hsa-miR-146a-5p gene PCR detection kit and application thereof
A detection kit and the technology of the kit, which are used in the determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problems of high cost, lack of effectiveness and coverage of detection methods, etc., to improve the accuracy and improve the sample. The effect of coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: hsa-miR-146a-5p gene PCR detection kit
[0041] Table 1 Composition of hsa-miR-146a-5p gene detection kit:
[0042]
[0043]
[0044] Wherein, the specific miRNA reverse transcription primer is hsa-miR-146a-5p specific miRNA reverse transcription primer and U6 specific miRNA reverse transcription primer, and the sequences are shown in Table 2.
[0045] Table 2
[0046] name
sequence
hsa-miR-146a-5p
5'–GTGCAGGGTCCGAGGTCAGAGCCACCTGGGCAATTTTTTTTTTTAACCCA-3'
U6
5'–GTGCAGGGTCCGAGGTCAGAGCCACCTGGGCAATTTTTTTTTTTAGTCAG-3'
[0047] Table 3 hsa-miR-146a-5p gene-specific primers (L1, R1) and probe (P1) sequences
[0048] name
sequence
Strand
T m
Purity
Modification
L1
CCGATGTGTATCTCAGCTTT
forward
54.7
gaps
R1
ATCCCAGCTGAAGAACTGAA
reverse
53.4
gaps
P1
AGGTCTGACACTGACACAACCCATGG
reverse
62.6
HPLC
5'Fam-3'Tamra
...
Embodiment 2
[0051] Example 2: Application of hsa-miR-146a-5p gene PCR detection kit
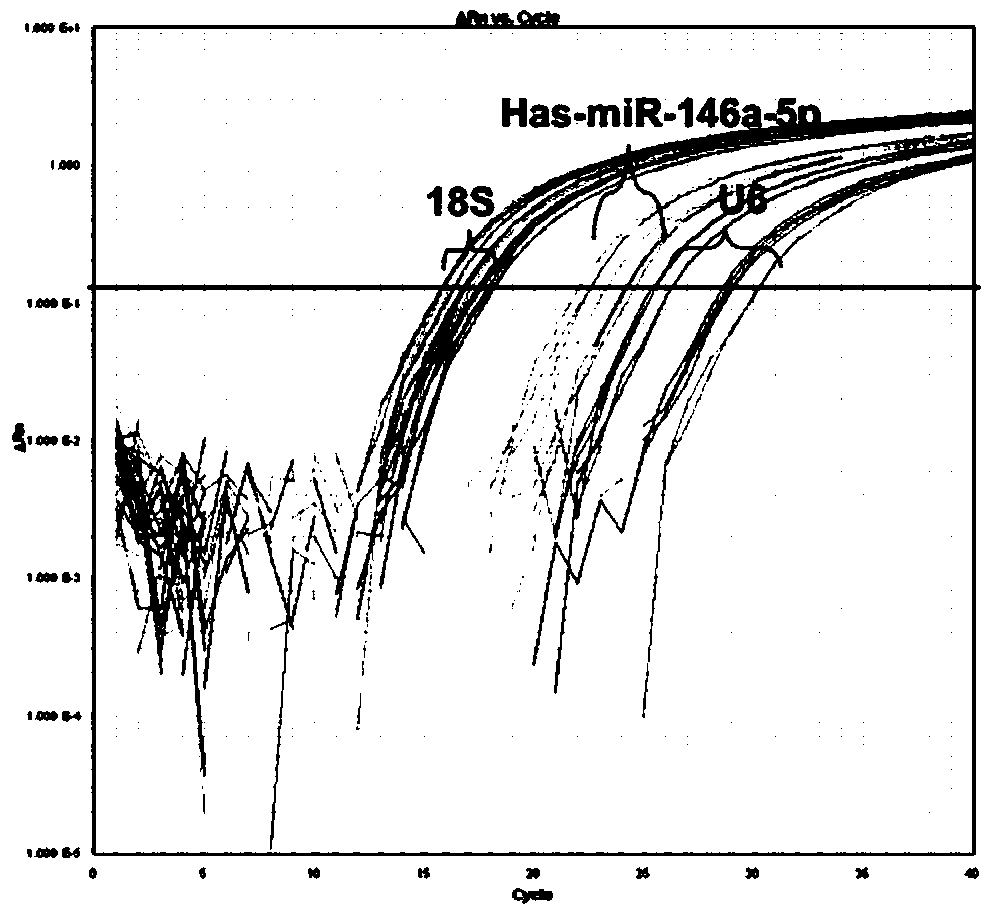
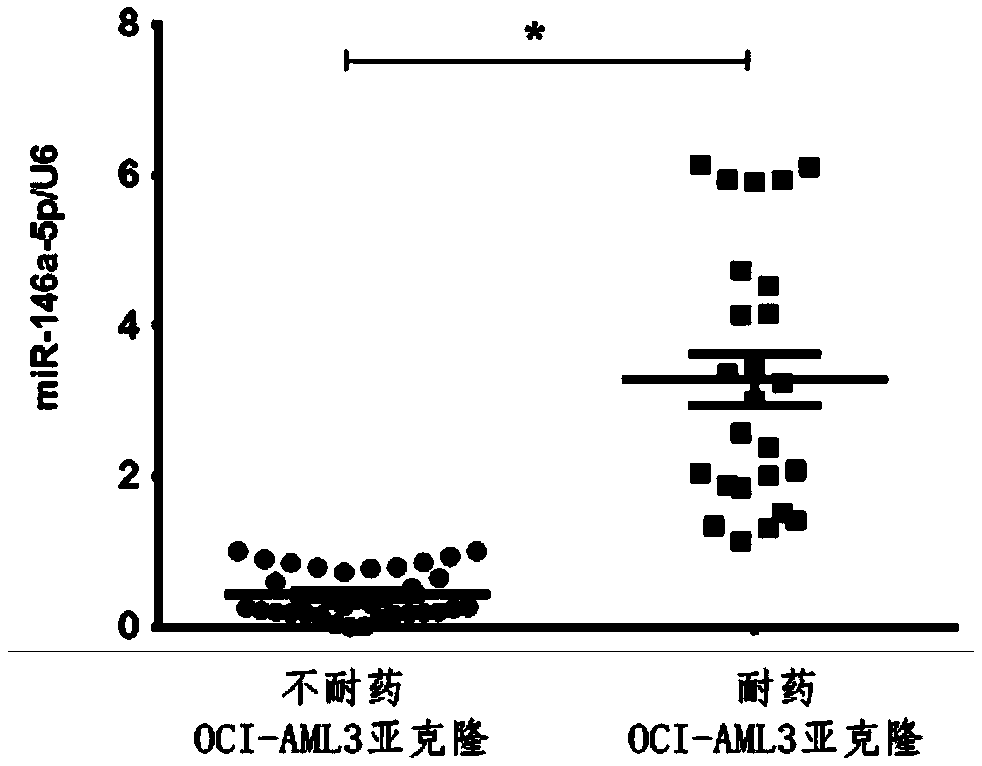
[0052] Using the hsa-miR-146a-5p gene PCR detection kit of Example 1, 61 cases of different drug-resistant subcloned samples of the acute myeloid leukemia cell line OCI-AML3 (purchased from ATCC) were detected by fluorescent RT-PCR Conduct research to determine the optimal cut-off value of hsa-miR-146a-5p in the evaluation of drug resistance analysis of acute myeloid leukemia cells.
[0053] 1. Preparation of subcloned samples with different drug resistance
[0054] OCI-AML3 cells were routinely cultured at 37 degrees 5% CO 2 In the incubator, the normal medium for the cells is RPMI1640 supplemented with 10% fetal bovine serum, the medium is changed every 72 hours, and the seeding density is 0.2×10 6 a / mL;
[0055] Collect OCI-AML3 cells in the logarithmic growth phase, according to 1×10 6 Cells / mL density inoculated in the normal medium containing 1mMAraC (cytarabine) and cultured for 48 hours, then...
Embodiment 3
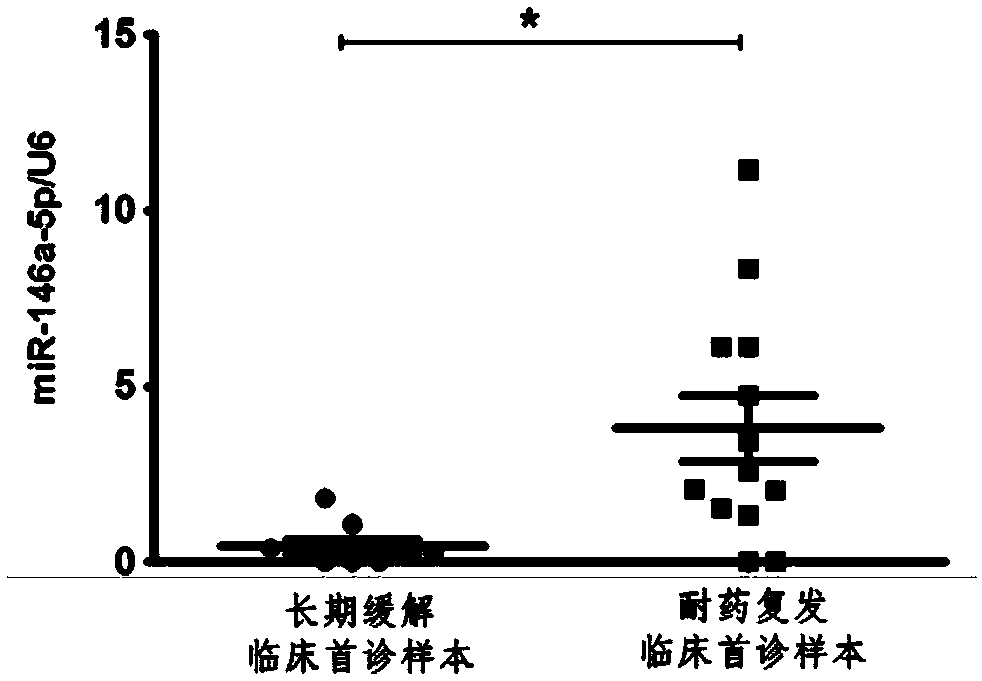
[0075] Embodiment 3: Reliability test of the optimal critical value of the hsa-miR-146a-5p gene PCR detection kit of the present invention in clinical samples
[0076] 1. Experimental method
[0077] (1) Test samples: According to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines: Acute Myeloid Leukemia Diagnostic Criteria (2015.V1), anticoagulated peripheral blood samples (before treatment) collected from first-diagnosed patients with acute myeloid leukemia 28 Cases (the first consultation time was July-September 2015). According to whether leukemia patients achieved long-term remission within 24 months after receiving treatment, the test samples were divided into two groups: remission group (15 cases) and drug-resistant relapse group (13 cases);
[0078] (2) Extract the total cellular RNA according to the standard instructions provided by the conventional method (QIAamp RNA Blood Mini kit), and store it at -80°C after the Nano drop detects the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com