Adrenocorticotrophic hormone assay kit and preparation method thereof
An adrenal cortex and kit technology, applied in the field of immunoassay, can solve the problems affecting the accuracy of results, poor stability of reagents, magnetic bead agglutination, etc., and achieve the effects of reliable detection means, good stability and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of embodiment 1 calibrator, quality control product
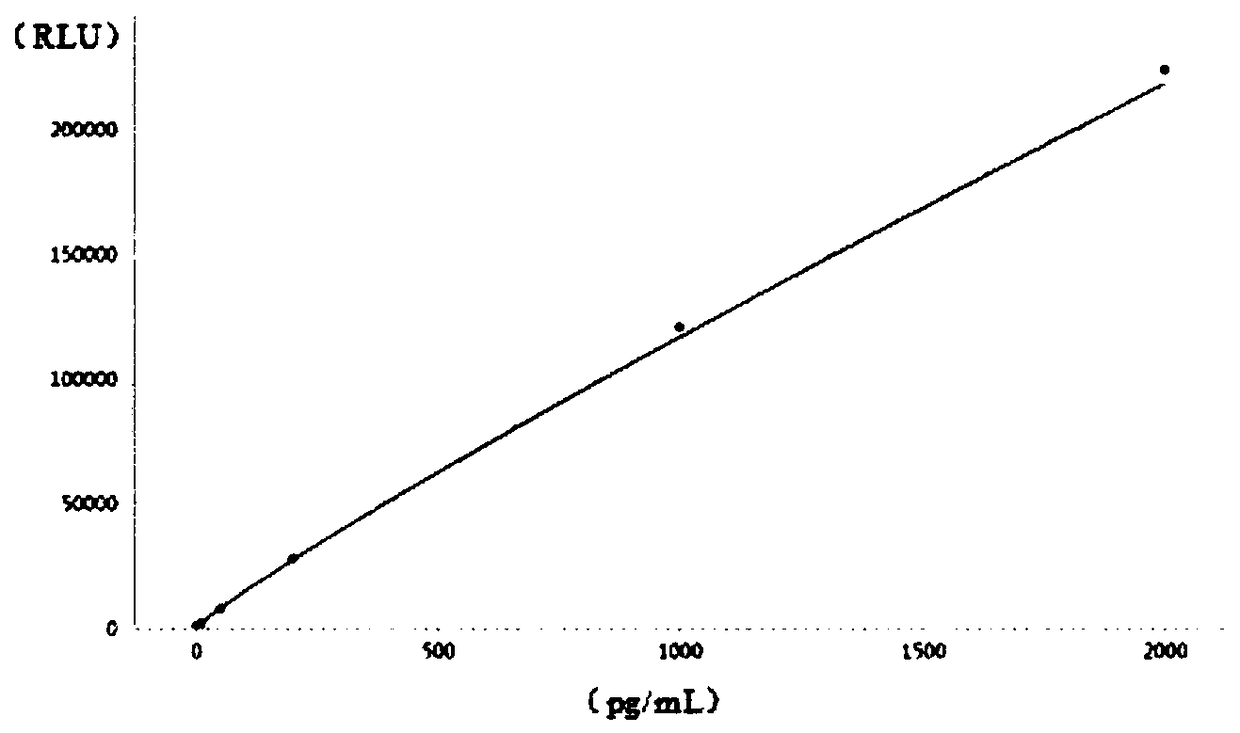
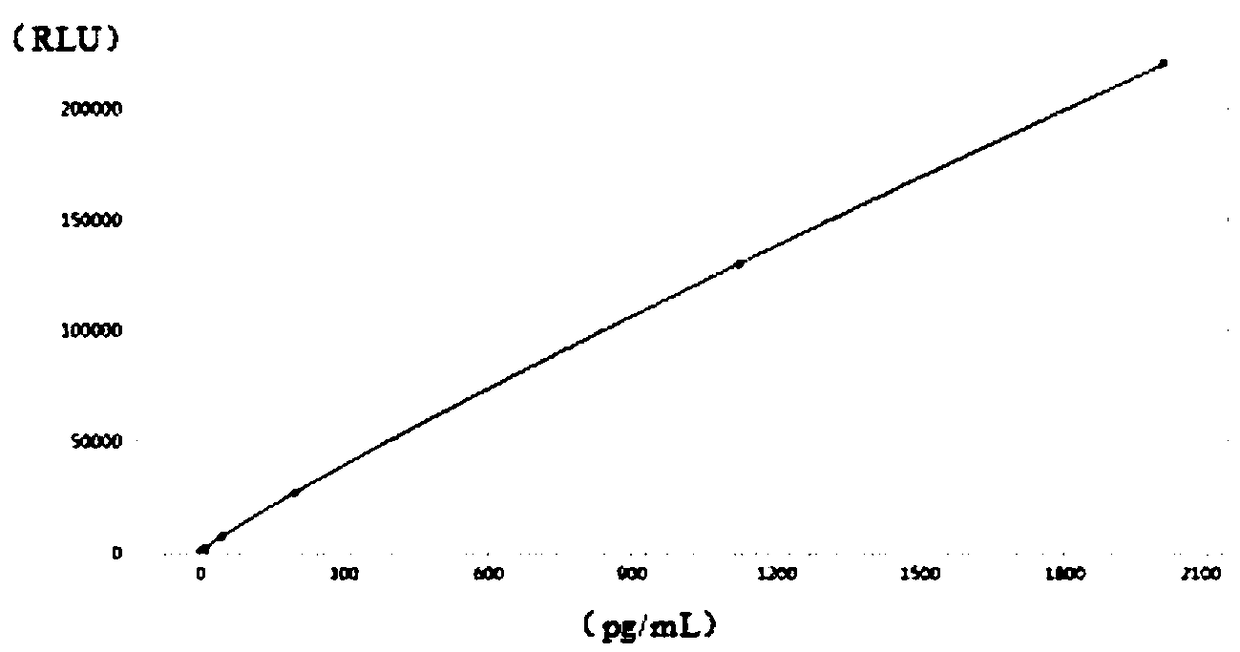
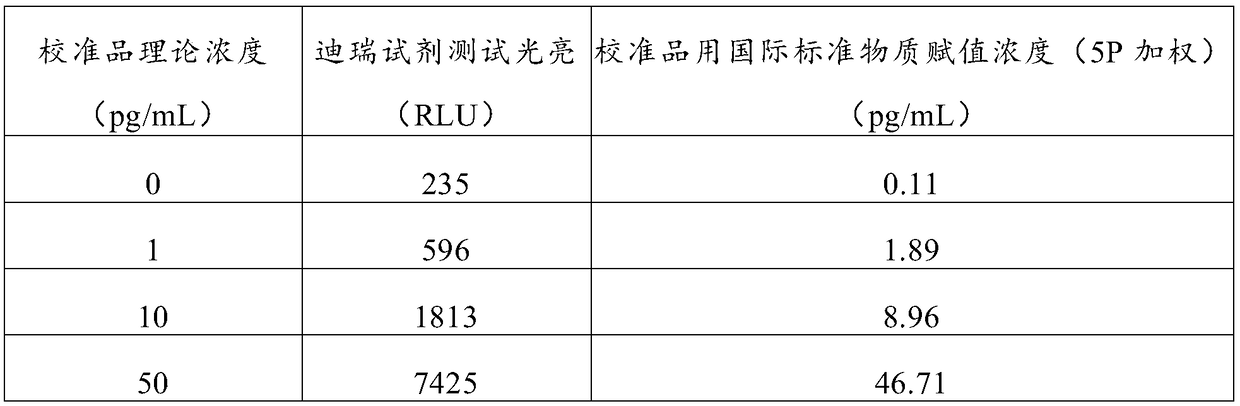
[0051] Take 1ml of 0.4μg / mL corticotropin stock solution, add it to 99ml calibrator buffer solution, and dilute to 4000pg / mL calibrator stock solution. Then dilute the calibrator mother solution in sequence to the concentration points of 2000pg / mL, 1000pg / mL, 200pg / mL, 50pg / mL, 10pg / mL, 1pg / mL, and use the calibrator matrix buffer as the concentration point of 0pg / mL point. The composition of the calibrator buffer: composed of 0.1mol / L PB, 0.15mol / L sodium chloride, 1% bovine serum albumin and 0.05% tween-20, pH value 6.5, stored at 2-8°C for later use .
[0052] Add 10ml of calibrator mother solution to 30ml of calibrator matrix buffer to prepare 40ml of high-concentration quality control with a concentration of 1000pg / mL.
[0053] Add 0.5ml of calibrator mother solution to 39.5ml calibrator matrix buffer solution to prepare 40ml of low-concentration quality control substance with a concentration ...
Embodiment 2
[0054] The preparation of embodiment 2 R1
[0055] Take the streptavidin magnetic particle solution with a concentration of 100 mg / ml, add 20 times the volume of TBST solution and mix well for 10 minutes, then place it on a magnetic separator until the supernatant is free of turbidity, discard the supernatant, and keep the magnetic Particles: After repeated washing 3 times, the solid-phase reagent R1 with a magnetic bead concentration of 0.03% was prepared with 50mM MES buffer solution containing 0.05% Tween-20 and 0.05% Proclin300, pH 6.5.
Embodiment 3
[0056] The preparation of embodiment 3 R1
[0057] Take the streptavidin magnetic particle solution with a concentration of 50 mg / ml, add 5 times the volume of TBST solution and mix well for 15 minutes, then place it on a magnetic separator until the supernatant is free of turbidity, discard the supernatant, and keep the magnetic Particles: after repeated washing 3 times, use 50mM MES buffer solution containing 0.05% Tween-20 and 0.05% Proclin300, pH 6.5 to prepare solid-phase reagent R1 with a magnetic bead concentration of 0.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com