Catalyst for preparing tetrafluoroethylene and hexafluoropropylene through catalytic cracking of trifluoromethane and preparation method thereof
A trifluoromethane, catalytic cracking technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of low catalyst conversion rate, easy coking and loss of catalyst It can improve the conversion rate and target product selectivity, improve the low temperature reaction activity, and improve the anti-carbon deposition ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
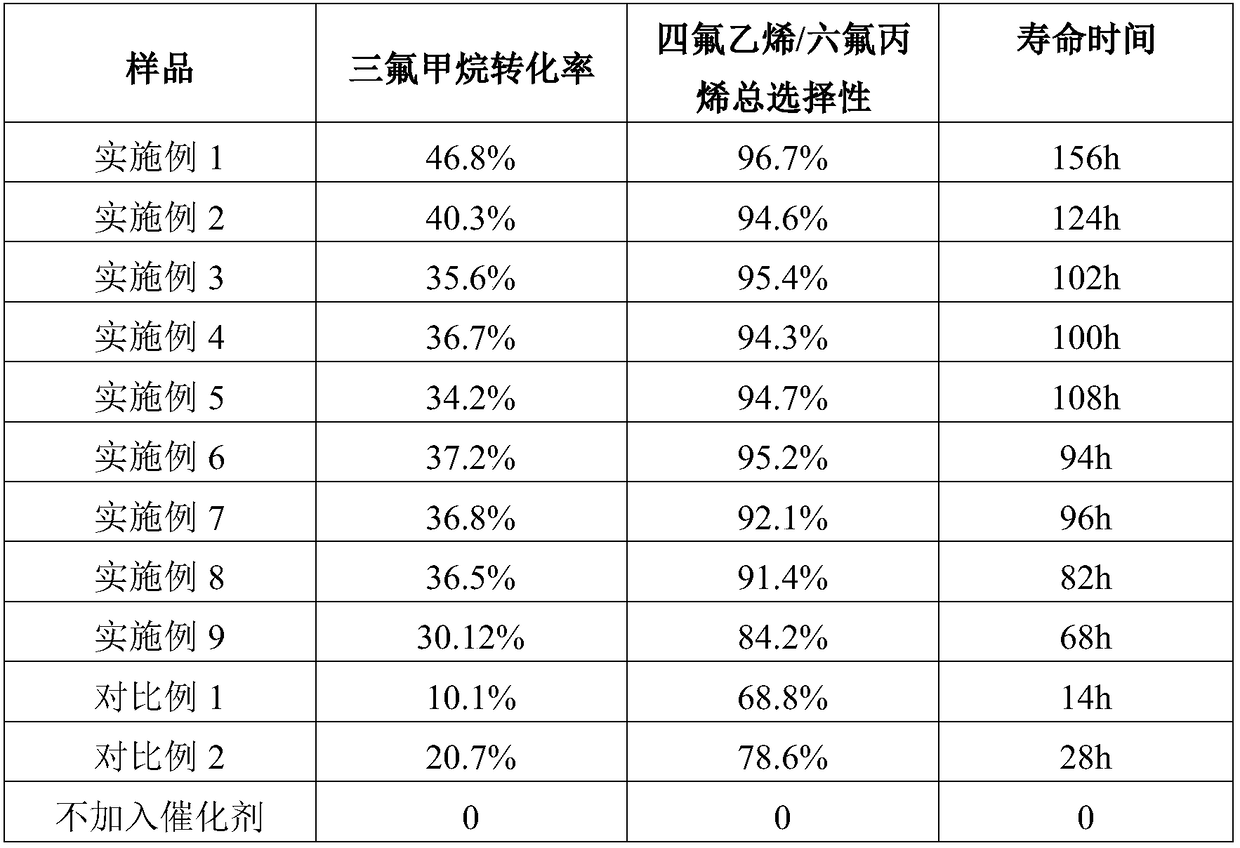
[0032] A preparation method for a catalyst for preparing tetrafluoroethylene and hexafluoropropylene by catalytic cracking of trifluoromethane, comprising the steps of:
[0033] Take Al 2 o 3 Carrier 100g, put into drying box, dry at 100°C for 3h; put 13.5g La(NO 3 ) 3 Dissolve in 100ml of pure water to make an impregnation solution; put the dried carrier into the impregnation solution, take it out after immersion for 20 minutes, put it in a drying oven and dry it at 100°C for 3 hours to obtain a precursor; take it out, put it in a roasting furnace, Calcined at 400°C for 6h in a nitrogen atmosphere. Take out the roasted precursor and put it into In the reaction tube, N 2 , N 2 The flow rate is 300ml / min, and it is dried at a temperature of 300°C for 24h. Then pass trifluoromethane gas at 300°C for fluorination at a flow rate of 6L / min, and turn off the nitrogen gas after 2h; pass trifluoromethane alone at a flow rate of 6L / min, and fluorinate at 350°C for 60h to finally ...
Embodiment 2
[0035] A preparation method for a catalyst for preparing tetrafluoroethylene and hexafluoropropylene by catalytic cracking of trifluoromethane, comprising the steps of:
[0036] Take AlF 3 Carrier 100g, put into drying box, dry at 100°C for 3h; put 13.5g La(NO 3 ) 3 Dissolve in 100ml of pure water to make an impregnation solution; put the dried carrier into the impregnation solution, take it out after immersion for 20 minutes, put it in a drying oven and dry it at 100°C for 3 hours to obtain a precursor; take it out, put it in a roasting furnace, Calcined at 400°C for 6h in a nitrogen atmosphere. Take out the roasted precursor and put it into In the reaction tube, N 2 , N 2 The flow rate is 300ml / min, and it is dried at a temperature of 300°C for 24h. Then pass trifluoromethane gas at 300°C for fluorination at a flow rate of 6L / min, and turn off the nitrogen gas after 2h; pass trifluoromethane alone at a flow rate of 6L / min, and fluorinate at 350°C for 60h to finally ob...
Embodiment 3
[0038] A preparation method for a catalyst for preparing tetrafluoroethylene and hexafluoropropylene by catalytic cracking of trifluoromethane, comprising the steps of:
[0039] Take Al 2 o 3 Carrier 100g, placed in a drying oven, dried at 100°C for 3 hours; 13.5g of CsNO 3 Dissolve in 100ml of pure water to make an impregnation solution; put the dried carrier into the impregnation solution, take it out after immersion for 20 minutes, put it in a drying oven and dry it at 100°C for 3 hours to obtain a precursor; take it out, put it in a roasting furnace, Calcined at 400°C for 6h in a nitrogen atmosphere. Take out the roasted precursor and put it into In the reaction tube, N 2 , N 2 The flow rate is 300ml / min, and it is dried at a temperature of 300°C for 24h. Then pass trifluoromethane gas at 300°C for fluorination at a flow rate of 6L / min, and turn off the nitrogen gas after 2h; pass trifluoromethane alone at a flow rate of 6L / min, and fluorinate at 350°C for 60h to fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com