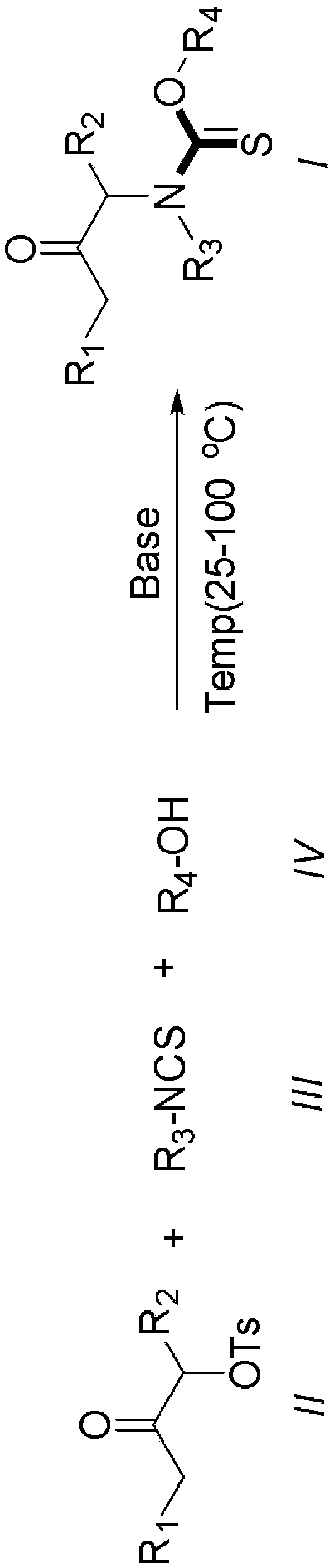
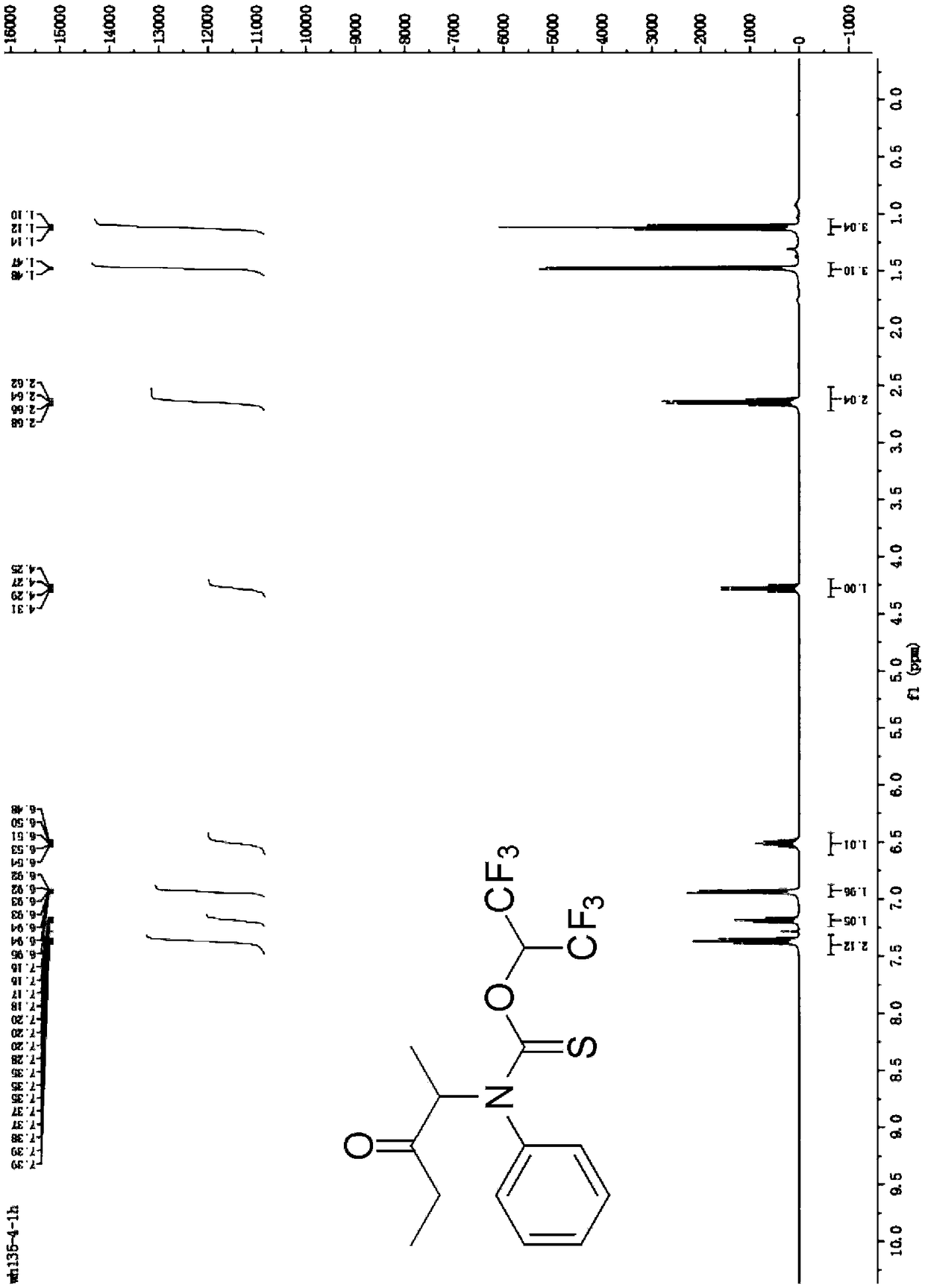
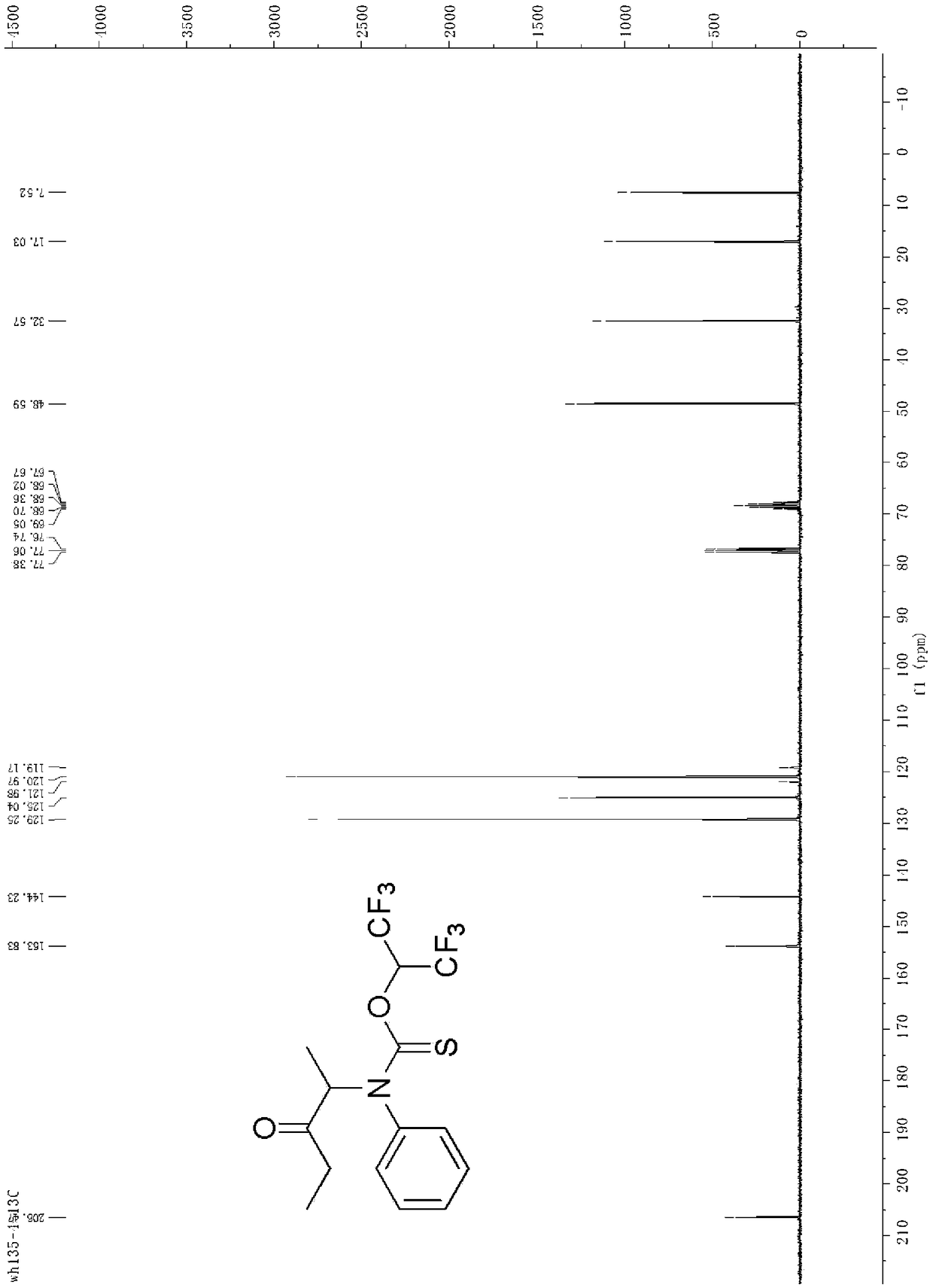
Fluorine-containing thiocarbamate compound and synthesizing method thereof
A technology for fluorine-containing thiocarbamate and ester compounds, applied in the direction of organic chemistry and the like, can solve the problems of few reports on the synthesis of thiothiocarbamate, achieve good industrial application prospects, high selectivity and yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of O-(1,1,1,3,3,3-hexafluoropropan-2-yl)(3-oxopentan-2-yl)(phenyl)carbamate
[0031] Add 1.0 mmol of 3-oxopentan-2-yl 4-methylbenzenesulfonate, 1.2 mmol of phenylthioisocyanate, 1.5 mmol of triethylamine, and then 2 mL of hexafluoroisopropyl to the reaction vessel Alcohol, reacted at room temperature, after the reaction, washed with aqueous solution, then extracted with an organic solvent, dried, concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain the target product with a yield of 89%.
Embodiment 2
[0033] Synthesis of O-(1,1,1,3,3,3-hexafluoropropan-2-yl)(5-oxonan-4-yl)(phenyl)carbamate
[0034] Add 1.0 mmol of 5-oxononan-4-yl 4-methylbenzenesulfonate, 1.2 mmol of phenylthioisocyanate, 1.5 mmol of triethylamine, and then 2 mL of hexafluoroisopropyl to the reaction vessel Alcohol, reacted at room temperature, after the reaction, washed with aqueous solution, then extracted with an organic solvent, dried, concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain the target product with a yield of 85%.
Embodiment 3
[0036] Synthesis of O-(1,1,1,3,3,3-hexafluoropropan-2-yl)(6-oxoundecan-5-yl)(phenyl)carbamate
[0037]Add 1.0 mmol of 6-oxoundecane-5-yl 4-methylbenzenesulfonate, 1.2 mmol of phenylthioisocyanate, 1.5 mmol of triethylamine, and then 2 mL of hexafluoroisocyanate into the reaction vessel Propanol was reacted at room temperature. After the reaction, it was washed with aqueous solution, then extracted with an organic solvent, dried, evaporated and concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain the target product with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com