UV light-cured organic silicon polyurethane acrylate monomer and preparation method thereof
A polyurethane acrylate and silicone technology, applied in the field of UV curing silicone monomer and its synthesis, can solve the problems of easy hydrolysis, unstable Si-O-C bond, etc., and achieve the effect of convenient adjustment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
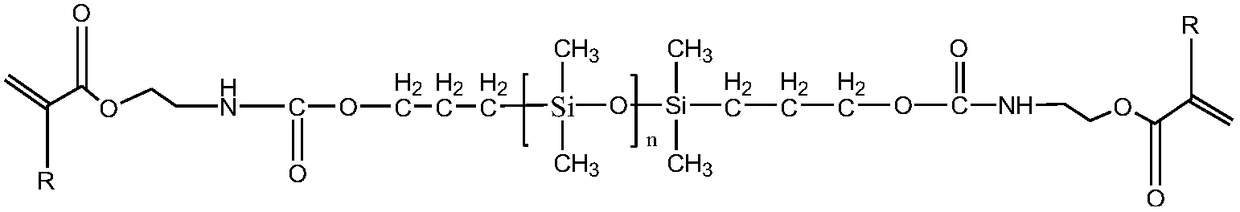
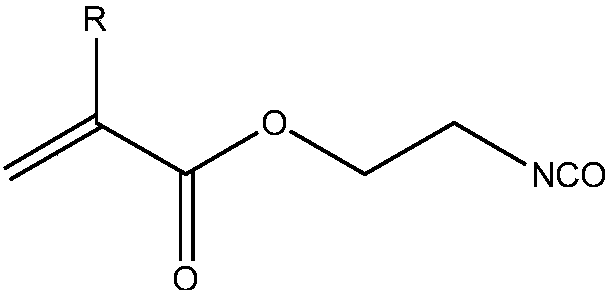
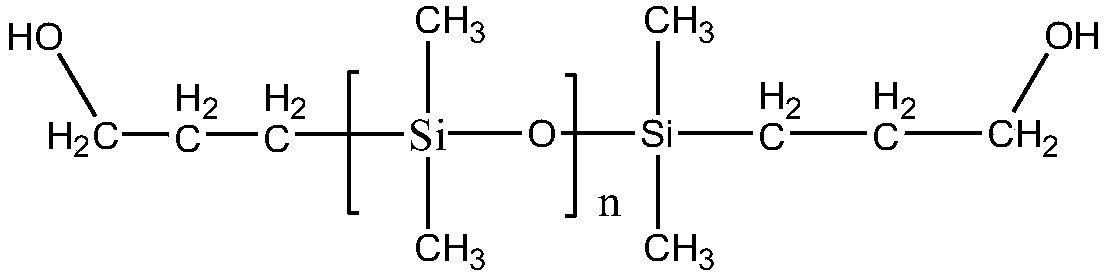
[0035] Add 50 g of hydroxyhydrocarbyl di-blocked polydimethylsiloxane with a degree of polymerization of 30, 100 g of n-heptane, 0.02 g of dibutyltin dilaurate, and 0.02 g of polymerization inhibitor BHT into a three-necked flask, and stir magnetically for half a day in an ice-water bath. After 1 hour, 9g of isocyanate ethyl methacrylate was added dropwise to the reaction solution, and the temperature was raised to 50°C for 4 hours. After the reaction, 3g of activated carbon was added, and magnetically stirred overnight. After filtration, rotary steaming, 55.96g of the product was obtained, and the product was protected from light. save.
Embodiment 2
[0037] Add 50 g of hydroxyhydrocarbyl di-blocked polydimethylsiloxane with a degree of polymerization of 40, 100 g of n-heptane, 0.02 g of dibutyltin dilaurate, and 0.02 g of polymerization inhibitor BHT into a three-necked flask, and stir magnetically for half a day in an ice-water bath. 6.5 g of isocyanate ethyl methacrylate was added dropwise to the reaction solution, and the temperature was raised at 40° C. for 5 hours of heat preservation reaction; light save.
Embodiment 3
[0039] Add 50 g of hydroxyhydrocarbyl di-blocked polydimethylsiloxane with a degree of polymerization of 30, 100 g of n-heptane, 0.02 g of dibutyltin dilaurate, and 0.02 g of polymerization inhibitor BHT into a three-necked flask, and stir magnetically for half a day in an ice-water bath. After 1 hour, 9g of ethyl isocyanate acrylate was added dropwise to the reaction solution, and the temperature was raised to 50°C for 6 hours of heat preservation reaction; after the reaction was completed, 4g of activated carbon was added, magnetically stirred overnight, filtered, and rotary evaporated to obtain 57.06g of the product, which was stored in the dark.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com