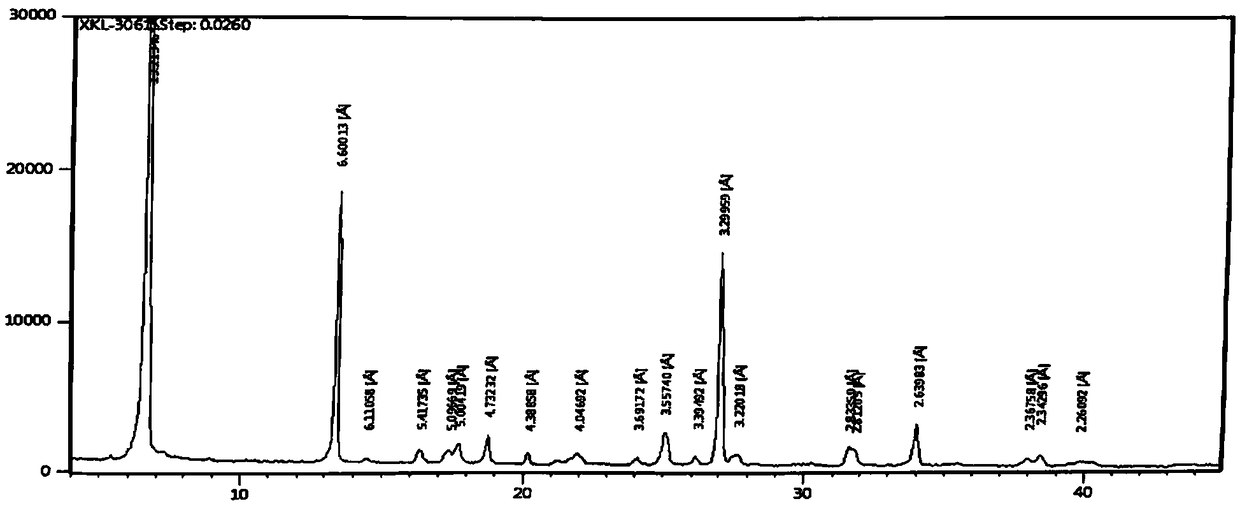
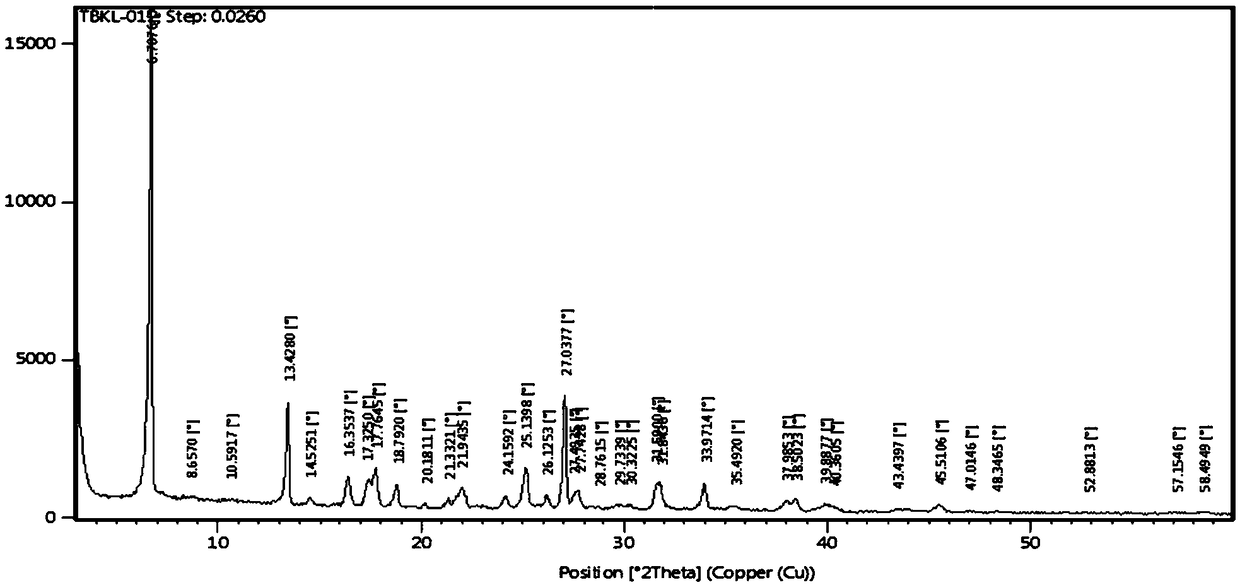
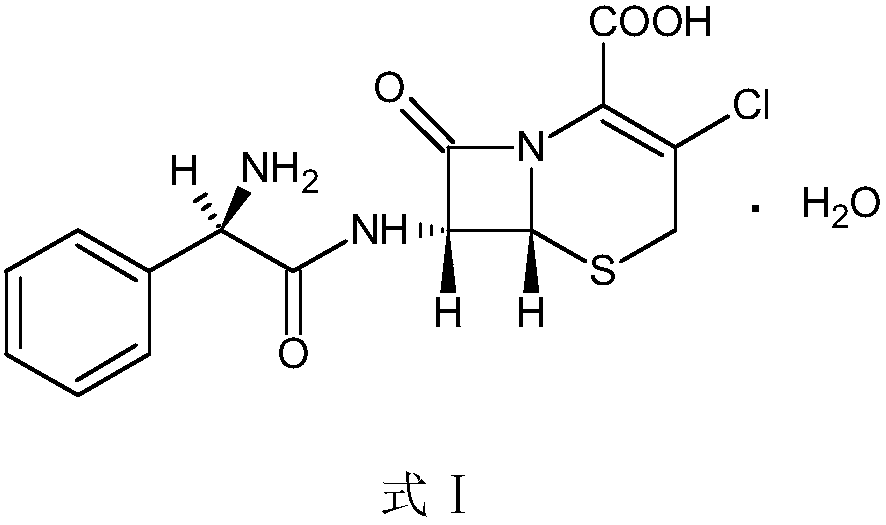
Preparation method of cefaclor suitable for industrial production
A technology of cefaclor and cephalosporin, which is applied in the field of preparation of cefaclor, can solve the problems such as poor effect of enzyme filtration, achieve the effects of reducing production cost and environmental protection expenditure, reducing pressure, and reducing degradation caused by excessive enzyme filtration time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 150g of purified water and 40g of 7-ACCA to the clean reaction flask, cool down to 0-5°C, and slowly add 10% sodium hydroxide solution until the solution is clear. Add 45g of immobilized penicillin acylase for synthesis, control the temperature at 5-15°C, add the pre-prepared 40g of PGM solution for about 1 hour, and maintain the pH of the reaction feed solution with sodium bicarbonate solution = 6.0-7.5. After adding PGM, the reaction reaches 7- ACCA residues meet the requirements.
[0046] After the reaction is qualified, use sodium hydroxide to adjust the pH of the reaction feed liquid to 8.0, the feed liquid is dissolved, pour the feed liquid into a specific device, and pass through an 80-mesh filter for separation under stirring. Cool the feed solution to 0-5°C, add activated carbon for decolorization for 30 minutes, filter, wash with purified water, and combine the filtrate and washing liquid into a crystallization bottle.
[0047] Slowly add hydrochloric aci...
Embodiment 2
[0049] Add 150g of purified water and 40g of 7-ACCA to the clean reaction flask, cool down to 0-5°C, and slowly add 10% sodium hydroxide solution until the solution is clear. Add 42g of immobilized penicillin acylase for synthesis, control the temperature at 5-15°C, and add 36.5g of the pre-prepared PGM solution for about 2 hours. The sodium bicarbonate solution maintains the pH of the reaction feed solution at 6.0-7.5, and the reaction reaches 7 after adding PGM. - ACCA residues are compliant.
[0050] After the reaction is qualified, use sodium hydroxide to adjust the pH of the reaction feed liquid to 8.5, the feed liquid is dissolved, pour the feed liquid into a specific device, and pass through an 80-mesh filter for separation under stirring. Cool the feed solution to 0-5°C, add activated carbon for decolorization for 30 minutes, filter, wash with purified water, and combine the filtrate and washing liquid into a crystallization bottle.
[0051]Hydrochloric acid solution ...
Embodiment 3
[0055] Add 150g of purified water and 40g of 7-ACCA to the clean reaction flask, cool down to 0-5°C, and slowly add 10% sodium hydroxide solution until the solution is clear. Add 45g of immobilized penicillin acylase for synthesis, control the temperature at 5-15°C, and slowly add the pre-prepared 41.2g PGM solution for about 3 hours, and the sodium bicarbonate solution to maintain the pH of the reaction feed solution = 6.0-7.5, and react to 7-ACCA residues meet the requirements.
[0056] After the reaction is qualified, use sodium hydroxide to adjust the pH of the reaction feed liquid to 9.0, the feed liquid is dissolved, pour the feed liquid into a specific device, and pass through an 80-mesh filter for separation under stirring. Cool the feed solution to 0-5°C, add activated carbon for decolorization for 30 minutes, filter, wash with purified water, and combine the filtrate and washing liquid into a crystallization bottle.
[0057] Add 0.2 g of seed crystals to the hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com