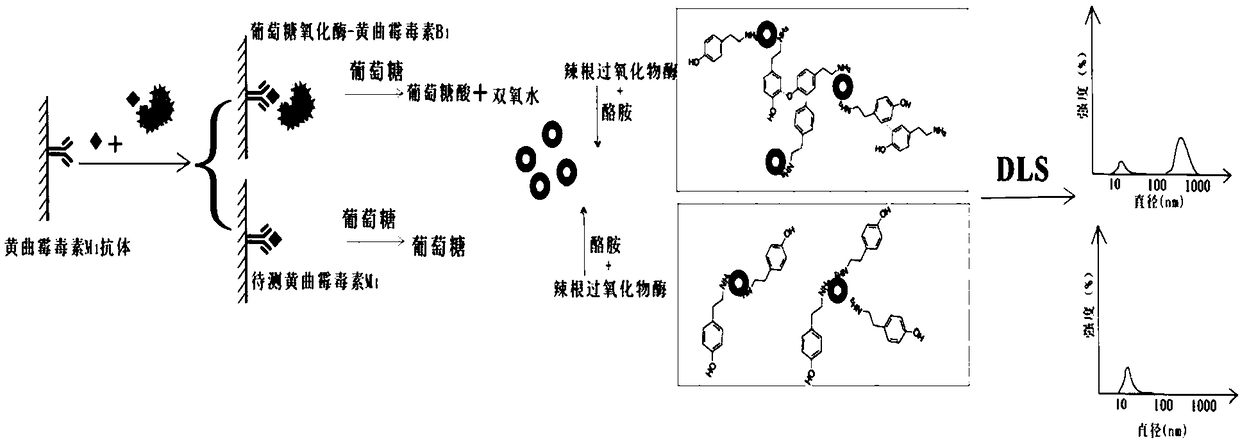
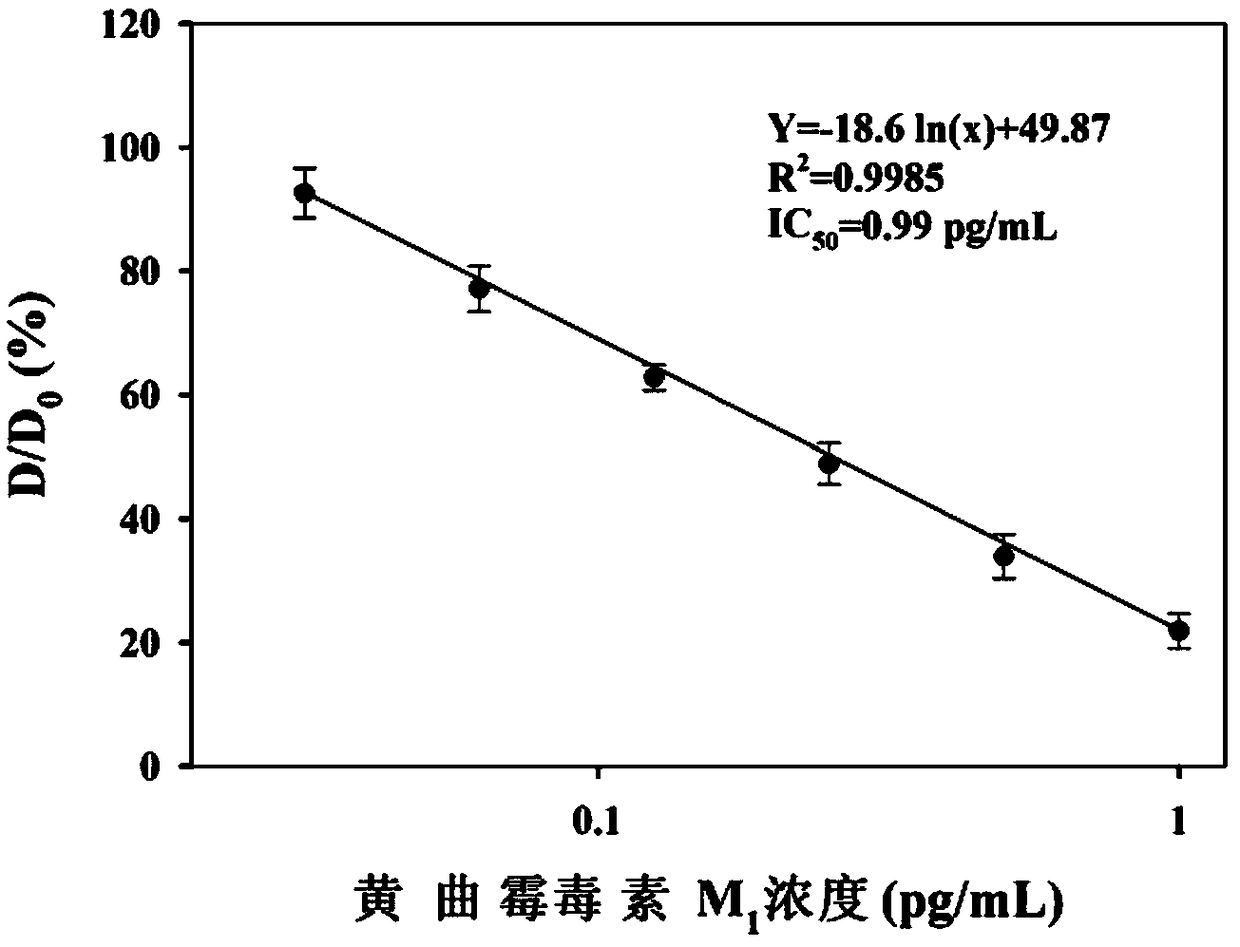
Method for detecting aflatoxin M1
An aflatoxin and reaction technology, which can be used in measurement devices, instruments, particle size analysis, etc., can solve the problems of low detection sensitivity, inability to meet detection requirements, and inability to meet the detection requirements of trace aflatoxin M, so as to reduce costs. , the effect of high molar extinction coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
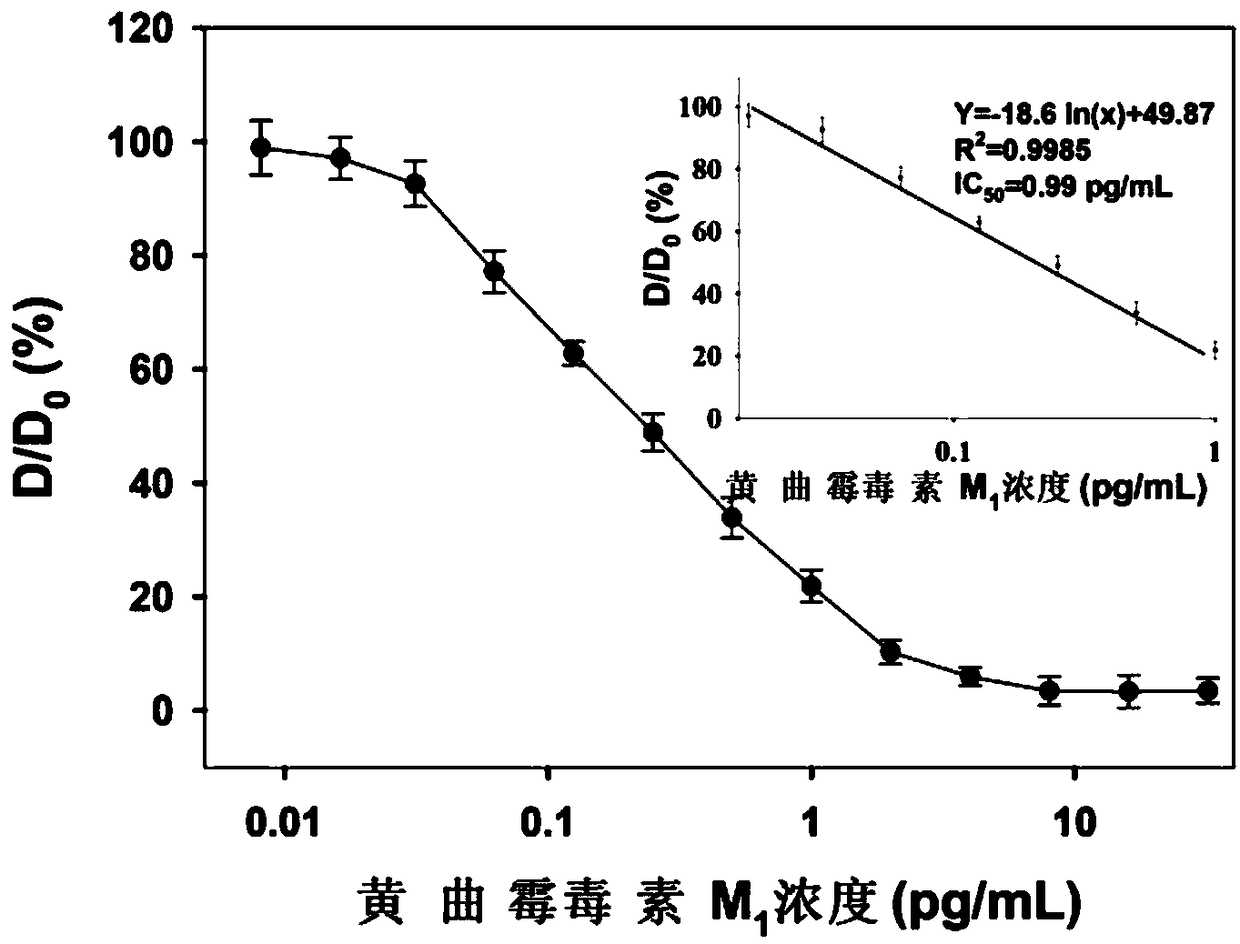
Image
Examples
Embodiment 1
[0106] The present embodiment provides a method of using glucose oxidase and aflatoxin B 1 Hapten Conjugated as Competing Antigen and Glucose as Substrate for Detection of Aflatoxin M in Milk by Direct Competitive ELISA Based on Dynamic Light Scattering 1 method of residue. The specific operation method is as follows:
[0107] (1) Formal test:
[0108] Dilute protein G to 20 μg / mL with 0.05 mol / L carbonate buffer solution with pH=9.6, and add 100 μL / well of the diluted solution to a 96-well microplate, and let it stand at 4°C for 12 hours; remove the enzyme label The liquid in the plate was washed three times with 0.01mol / L PBS containing 0.05% Tween 20, and then washed once again with 0.01mol / L PBS; 0.01mol / L pH=7.4 Anti-aflatoxin M at a concentration of 0.1 μg / mL diluted in phosphate buffer 1 For monoclonal antibody, let stand at 37°C for 1 hour; remove the liquid in the microtiter plate, wash the microtiter plate three times with 0.01mol / L PBS containing 0.05% Tween 20,...
Embodiment 2
[0120] The present embodiment provides a method of using glucose oxidase and aflatoxin B 1 Hapten Conjugated as Competing Antigen and Glucose as Substrate for Detection of Aflatoxin M in Milk by Direct Competitive ELISA Based on Dynamic Light Scattering 1 method of residue. The specific operation method is as follows:
[0121] (1) Formal test:
[0122]Dilute protein G to 18 μg / mL with 0.04 mol / L carbonate buffer solution with pH=9.4, and add 100 μL / well of the diluted solution to a 96-well microplate, and let it stand at 0°C for 8 hours; remove the enzyme label The liquid in the plate was washed three times with 0.01mol / L PBS containing 0.01% Tween 20, and then washed once again with 0.01mol / L PBS; 0.01mol / L pH=7.4 Anti-aflatoxin M at a concentration of 0.5 μg / mL diluted in phosphate buffer 1 For monoclonal antibody, stand at 35°C for 2 hours; remove the liquid in the microtiter plate, wash the microtiter plate three times with 0.01mol / L PBS containing 0.01% Tween 20, and ...
Embodiment 3
[0134] The present embodiment provides a method of using glucose oxidase and aflatoxin B 1 Hapten Conjugated as Competing Antigen and Glucose as Substrate for Detection of Aflatoxin M in Milk by Direct Competitive ELISA Based on Dynamic Light Scattering 1 method of residue. The specific operation method is as follows:
[0135] (1) Formal test:
[0136] Dilute protein G to 22 μg / mL with 0.06 mol / L carbonate buffer solution of pH=9.8, and add 100 μL / well of the diluted solution to a 96-well microtiter plate, and let it stand at 8°C for 12 hours; remove the enzyme label The liquid in the plate was washed three times with 0.01mol / L PBS containing 0.06% Tween 20, and then washed once again with 0.01mol / L PBS; 0.01mol / L pH=7.4 Anti-aflatoxin M diluted in phosphate buffer at a concentration of 1 μg / mL 1 For monoclonal antibody, stand at 39°C for 3 hours; remove the liquid in the microtiter plate, wash the microtiter plate three times with 0.01mol / L PBS containing 0.06% Tween 20, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com