Raltitrexed-related substance F and preparation and applications thereof
A technology of raltitrexed, related substances, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of N-(5-methylamino-2-thenoyl)-L-glutamic acid diethyl ester
[0047] Add 10g of 5-nitro-2-thiophenecarboxylic acid and 20ml of thionyl chloride into the reaction flask, heat and reflux for 3 hours, transfer the reaction solution to a single-necked flask, concentrate until no liquid flows out, and dissolve it with 30ml of dichloromethane. . Add 13.8g of L-glutamic acid diethyl ester, 20.6g of triethylamine, and 50ml of dichloromethane into a 150ml three-necked flask, stir and cool to 0-5°C, slowly add the dichloromethane solution of the concentrate dropwise. After dripping, the reaction is kept for 1 hour. After the reaction is over, wash with 100ml of 2mol / L hydrochloric acid and 100ml of saturated sodium bicarbonate solution in sequence. The organic phase was separated, dried over anhydrous sodium sulfate, and after concentration was completed, 16.53 g of N-(5-nitro-2-thenoyl)-L-glutamic acid diethyl ester was obtained, with a yield of 79.9%.
[0...
Embodiment 2
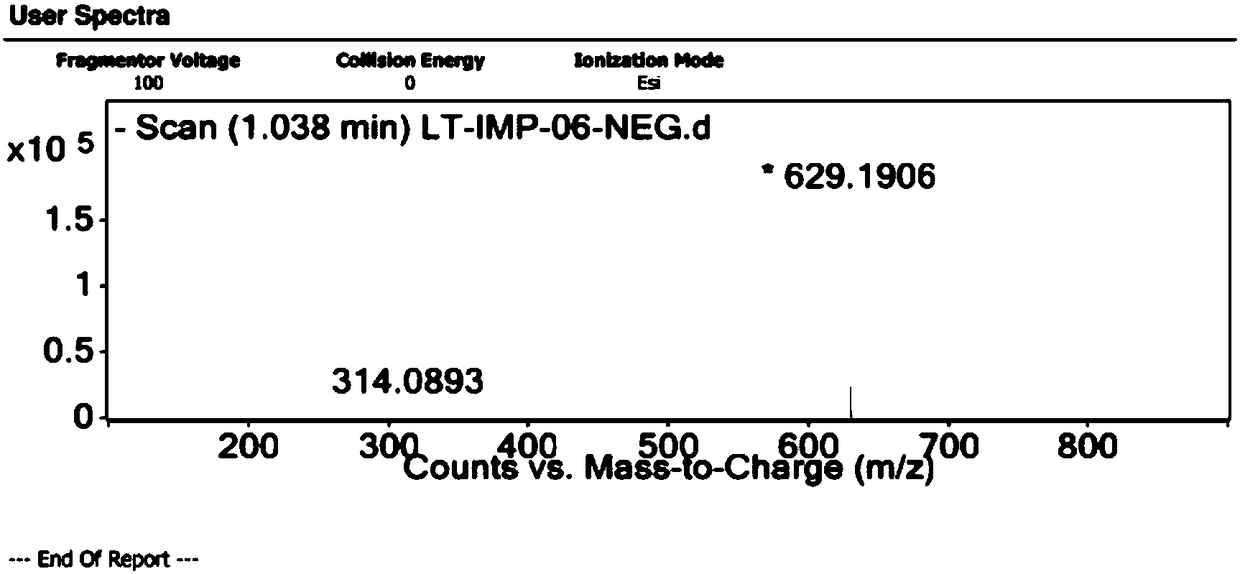
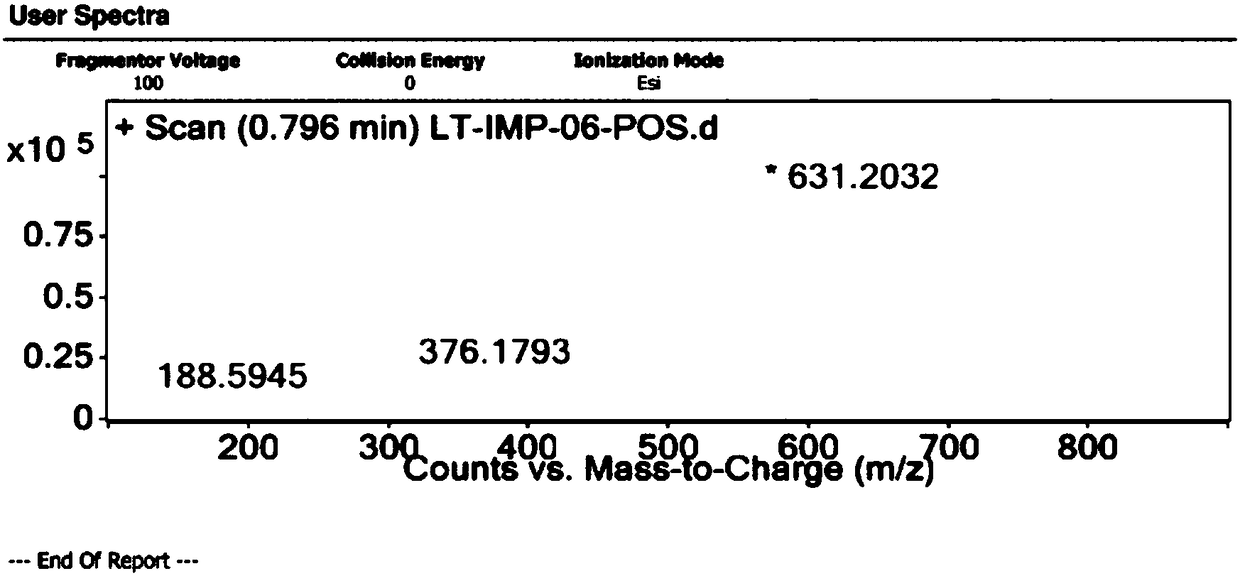
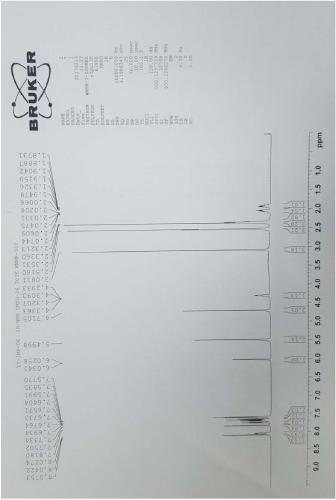
[0050] Example 2 Preparation of related substance F
[0051] Take 3.42g of N-(5-methylamino-2-thenoyl)-L-glutamic acid diethyl ester and dissolve it with 52ml of N,N-dimethylformamide, and prepare the solution for later use. Add 7.6g of 6-bromomethyl-3,4-dihydro-2-methyl-quinazolin-4-one and 38ml of N,N-dimethylformamide into a 250ml three-necked flask, stir and heat to 85 After reaching temperature, 4.88g cesium carbonate was added. After stirring for 10 minutes, the solution of N-(5-methylamino-2-thenoyl)-L-glutamic acid diethyl ester was added dropwise. React at 85±5℃ for 6 hours. After the reaction is over, the temperature is lowered to 10-30°C, suction filtered until no liquid flows out, and the filtrate is washed twice with 300 ml of 15% sodium chloride aqueous solution. Separate the water phase and extract twice with 150 ml of dichloromethane. Combine the organic phases, add anhydrous magnesium sulfate, stir and dehydrate for 2 hours, and filter with suction until no l...
Embodiment 3
[0053] Example 3 Preparation of related substance F
[0054] Take 3.42g of N-(5-methylamino-2-thenoyl)-L-glutamic acid diethyl ester and dissolve it with 52ml of N,N-dimethylacetamide, and prepare the solution for later use. Add 7.6g of 6-bromomethyl-3,4-dihydro-2-methyl-quinazolin-4-one and 38ml of N,N-dimethylacetamide into a 250ml three-necked flask, stir and heat to 85 After reaching temperature, 4.88g cesium carbonate was added. After stirring for 10 minutes, the solution of N-(5-methylamino-2-thenoyl)-L-glutamic acid diethyl ester was added dropwise. The temperature is controlled at 55±5°C for 6 hours. After the reaction is over, the temperature is lowered to 10-30°C, suction filtered until no liquid flows out, and the filtrate is washed twice with 300 ml of 15% sodium chloride aqueous solution. Separate the water phase and extract twice with 150 ml of dichloromethane. Combine the organic phases, add anhydrous magnesium sulfate, stir and dehydrate for 2 hours, and filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com