Quinazoline compound and preparation method and application thereof
A compound, quinazoline technology, applied in the direction of organic chemical methods, chemical instruments and methods, isotope introduction into organic compounds, etc., can solve the problems of low radiochemical yield, insufficient tumor selectivity, complex and cumbersome preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
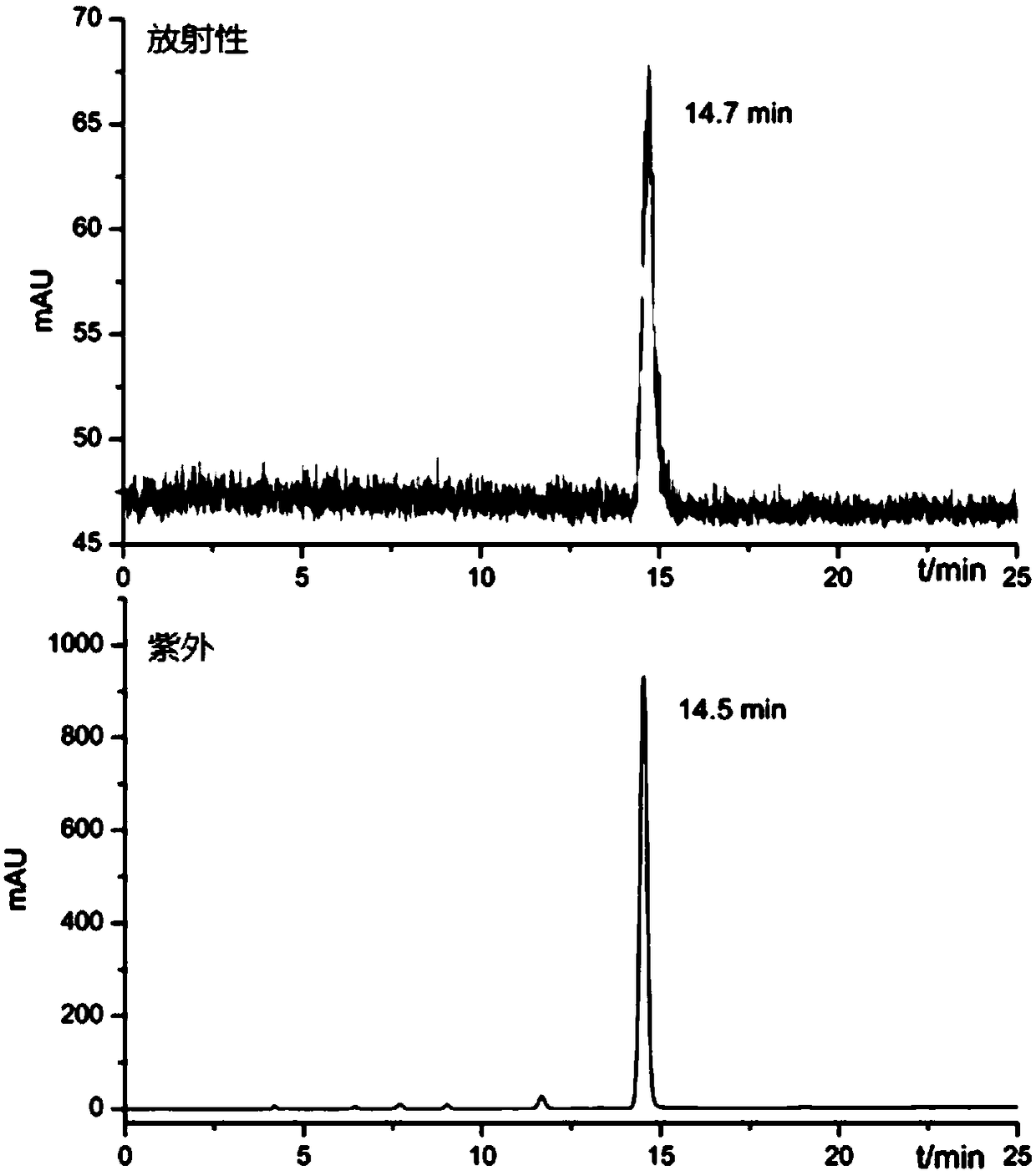
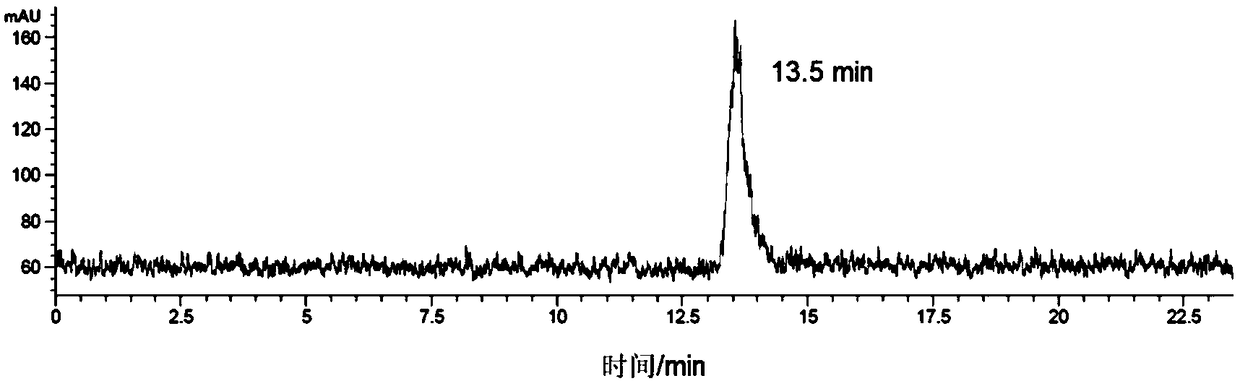
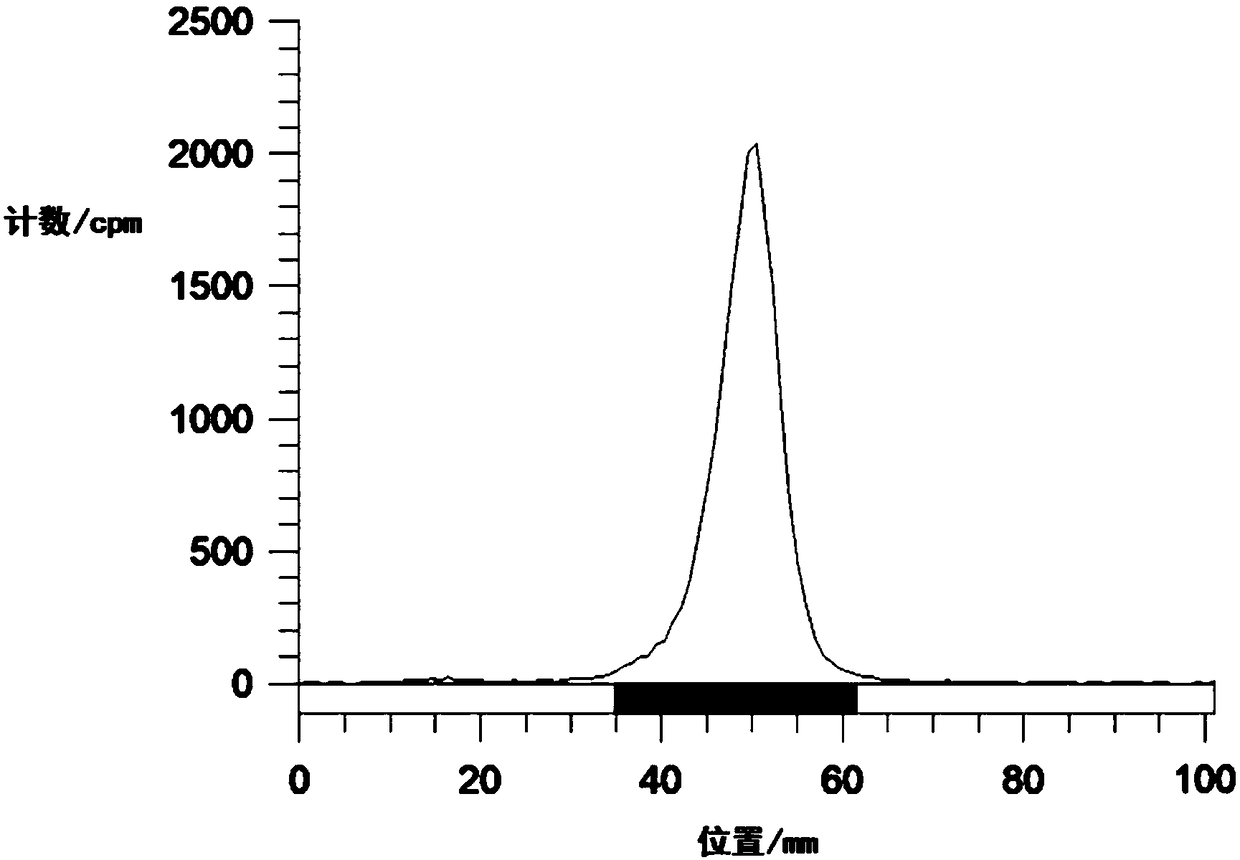
Image
Examples
Embodiment 1
[0067] Embodiment 1: the synthesis of I-1
[0068] (1) 100mCi 18 f - By quaternary ammonium type anion column QMA (the product of American Waters company, 18 f - Provided by Kexing Pharmaceutical Co., Ltd.) after capture, take 2mL K 222 (i.e. Kryptofix 222) solution (28.8mg K 222 , 6.0 mg K 2 CO 3 , 1920μL acetonitrile, 80μL water solution) will 18 Rinse F into the reaction bottle, immerse the reaction bottle in an oil bath at 95°C, dry it with nitrogen, then add 500 μL of anhydrous acetonitrile to dry it, repeat the drying with anhydrous acetonitrile twice;
[0069] (2) 500 μL of anhydrous acetonitrile solution containing 5 mg 2-azidoethyl p-toluenesulfonate was rapidly added to the solution containing K 222 、K 2 CO 3 and 18 f - In the mixture, stop the reaction after closed reaction at 95°C for 10 minutes, and cool in an ice-water bath to obtain compound C1-1, and the labeling rate can reach more than 97%;
[0070]
[0071] (3) The reaction liquid containing ...
Embodiment 2
[0075] Embodiment 2: the synthesis of I-1
[0076] (1) 40mCi 18 f - By quaternary ammonium type anion column QMA (the product of American Waters company, 18 f - Provided by Shanghai Kexing Pharmaceutical Co., Ltd.) after capture, take 1.0mL K 222 (i.e. Kryptofix 222) solution (14.4mg K2.2.2, 3.0mgK 2 CO 3 , 960μL acetonitrile, 40μL water solution) will 18 f - Rinse into the reaction bottle, immerse the reaction bottle in a 95°C oil bath, blow dry with nitrogen, then add 500 μL of anhydrous acetonitrile to dry, repeat the above operation twice;
[0077] (2) 400 μL of anhydrous dimethylformamide solution containing 5 mg 2-azidoethyl p-toluenesulfonate was rapidly added to the solution containing K 222 、K 2 CO 3 and 18 f - In the mixture, stop the reaction after closed reaction at 100°C for 5 minutes, and cool in an ice-water bath to obtain compound C1-1, and the labeling rate can reach more than 98%;
[0078]
[0079] (3) Add 500 μL of acetonitrile to the reactio...
Embodiment 3
[0083] Embodiment 3: the synthesis of I-2
[0084] (1) 4.23g 2-fluoroethyl-4 methylbenzenesulfonate and 3.78g sodium azide were reacted at room temperature in DMF for 12h, and the DMF solution of C2-1 was obtained after the solid was removed by filtration. The product was not further purified and directly for subsequent reactions;
[0085]
[0086] (2) Add 200 uL each of 0.5 mol / L copper sulfate and 1.5 mol / L sodium ascorbate to 6 mL of PBS (PH=6), then add 26.7 mg of icotinib and 2 mL of the above-mentioned C2-1 in DMF, 50 ℃ water bath reaction for 1h. The product was diluted with water, extracted with ethyl acetate, dried, and concentrated under reduced pressure to remove ethyl acetate to obtain product I-2 with a yield of 96%.
[0087]
[0088] The identification data of I-2 are as follows:
[0089] MS(ESI):M(C 24 h 25 FN 6 o 4 )=480.49(m / z),[M+H] + 481.15,[M+Na] + 503.15.
[0090] 1 H-NMR (400MHz, CD 3 OD):δ8.65(s,1H),8.54(s,1H),8.34(s,1H),8.22(s,1H),7.93...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com