Manganese-doped orange germanium stannate long afterglow fluorescent powder and preparation method thereof
A long-lasting phosphor and manganese-doped technology, which is applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of lack of red long-lasting materials doped with transition metals, and achieve environmental harmlessness, wide application, and preparation The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
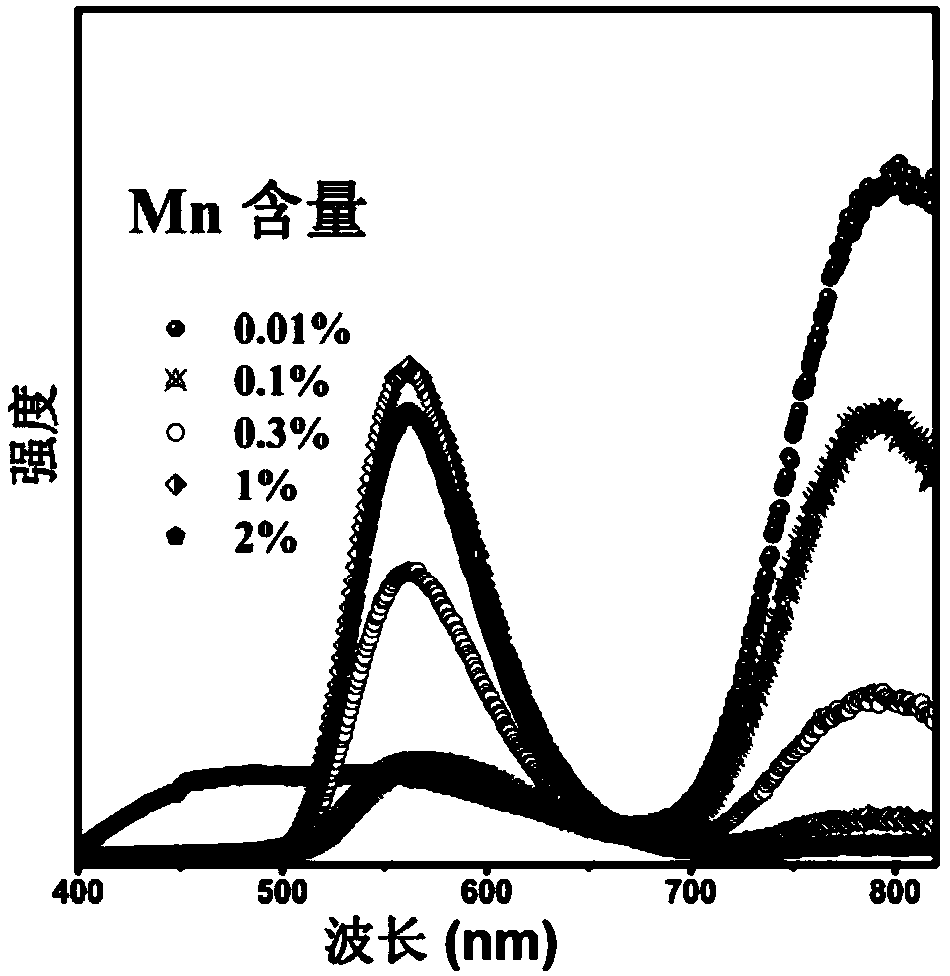
[0029] Select sodium carbonate, calcium carbonate, tin dioxide, germanium dioxide, and manganese carbonate as starting compound raw materials, and weigh four kinds of compound raw materials respectively according to the molar ratio of each element, totally 10 groups, and the proportioning ratio is as follows:
[0030] (1) Na:Ca:Sn:Ge:O:Mn=2:1:2.0000:3:12:0.0000, corresponding to x=0.0%;
[0031] (2) Na:Ca:Sn:Ge:O:Mn=2:1:1.9999:3:12:0.0001, corresponding to x=0.01%;
[0032] (3) Na:Ca:Sn:Ge:O:Mn=2:1:1.9995:3:12:0.0005, corresponding to x=0.05%;
[0033] (4) Na:Ca:Sn:Ge:O:Mn=2:1:1.9990:3:12:0.0010, corresponding to x=0.10%;
[0034] (5) Na:Ca:Sn:Ge:O:Mn=2:1:1.9980:3:12:0.0020, corresponding to x=0.20%;
[0035] (6) Na:Ca:Sn:Ge:O:Mn=2:1:1.9970:3:12:0.0030, corresponding to x=0.30%;
[0036] (7) Na:Ca:Sn:Ge:O:Mn=2:1:1.9950:3:12:0.0050, corresponding to x=0.50%;
[0037] (8) Na:Ca:Sn:Ge:O:Mn=2:1:1.9900:3:12:0.0100, corresponding to x=1.00%;
[0038] (9) Na:Ca:Sn:Ge:O:Mn=2:1:1....
Embodiment 2
[0047] Select sodium carbonate, calcium carbonate, tin dioxide, germanium dioxide, and manganese carbonate as the starting compound raw materials, and weigh four kinds of compound raw materials respectively according to the molar ratio of each element, totally 5 groups, and the proportioning ratio is as follows:
[0048] (1) Na:Ca:Sn:Ge:O:Mn=2:1:2.0000:3:12:0.0000, corresponding to x=0.0%;
[0049] (2) Na:Ca:Sn:Ge:O:Mn=2:1:1.9995:3:12:0.0005, corresponding to x=0.05%;
[0050] (3) Na:Ca:Sn:Ge:O:Mn=2:1:1.9980:3:12:0.0020, corresponding to x=0.20%;
[0051] (4) Na:Ca:Sn:Ge:O:Mn=2:1:1.9950:3:12:0.0050, corresponding to x=0.50%;
[0052] (5) Na:Ca:Sn:Ge:O:Mn=2:1:1.9850:3:12:0.0150, corresponding to x=1.50%;
[0053] After the mixture is ground and mixed, put it into a corundum crucible, place the crucible in a corundum boat, and put it into a high-temperature box-type electric furnace. Strictly control the heating rate, under the air at the temperature T (T = 1000, 1050, 1100, ...
Embodiment 3
[0056] Choose sodium nitrate, calcium carbonate, tin dioxide, germanium dioxide, and manganese carbonate as starting compound raw materials, and take four kinds of compound raw materials respectively according to the molar ratio of each element, totally 2 groups, and the proportioning ratio is as follows:
[0057] (1) Na:Ca:Sn:Ge:O:Mn=2:1:1.9995:3:12:0.0005, corresponding to x=0.05%;
[0058] (2) Na:Ca:Sn:Ge:O:Mn=2:1:1.9980:3:12:0.0020, corresponding to x=0.20%;
[0059] After the mixture is ground and mixed, put it into a corundum crucible, place the crucible in a corundum boat, and put it into a high-temperature box-type electric furnace. The heating rate is strictly controlled, and the calcining time is t=5h at the temperature T (T=1100, 1150, 1200, 1250°C) under the air. After cooling down to room temperature with the furnace, grind to obtain manganese-doped orange germanium stannate long-lasting phosphor.
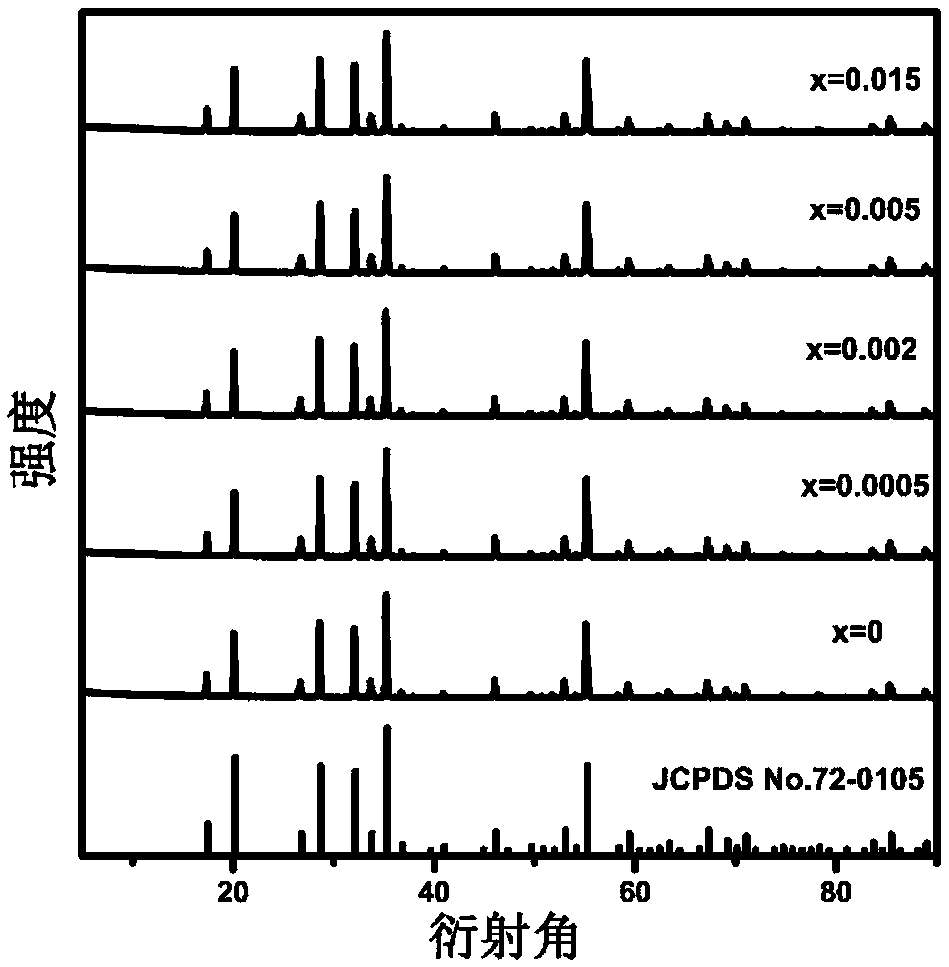
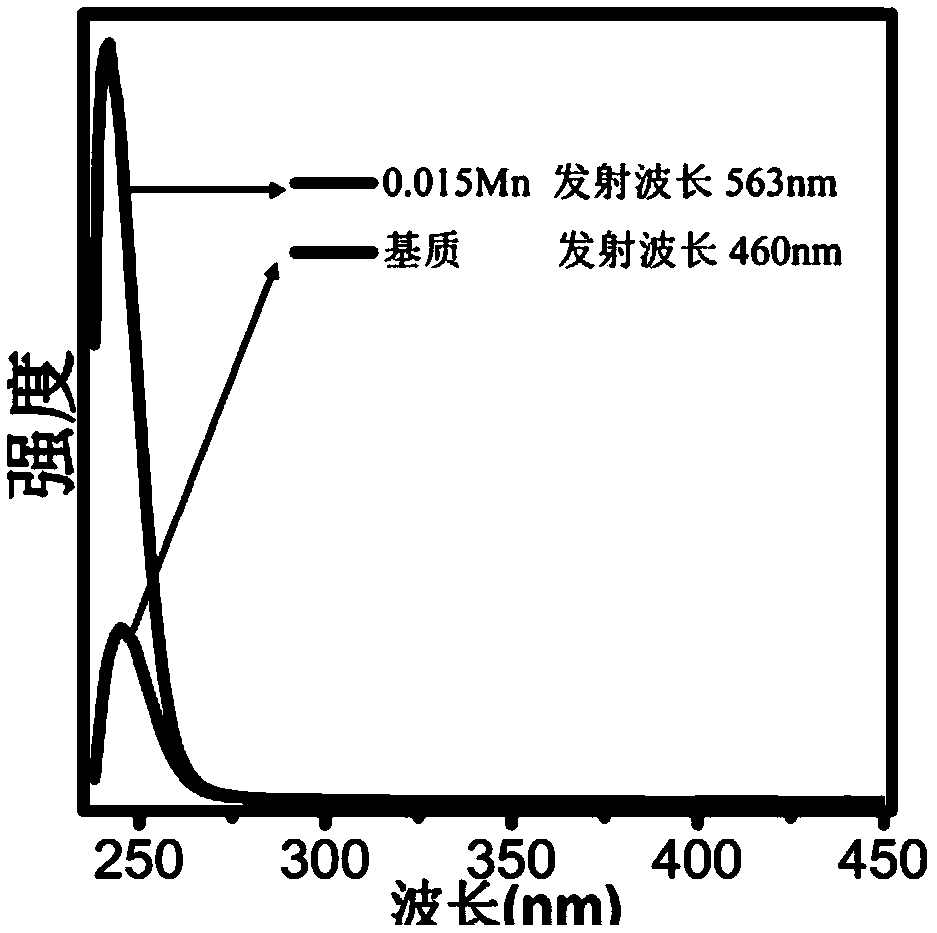
[0060] X-ray diffraction analysis showed that it was Na 2 CaSn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com