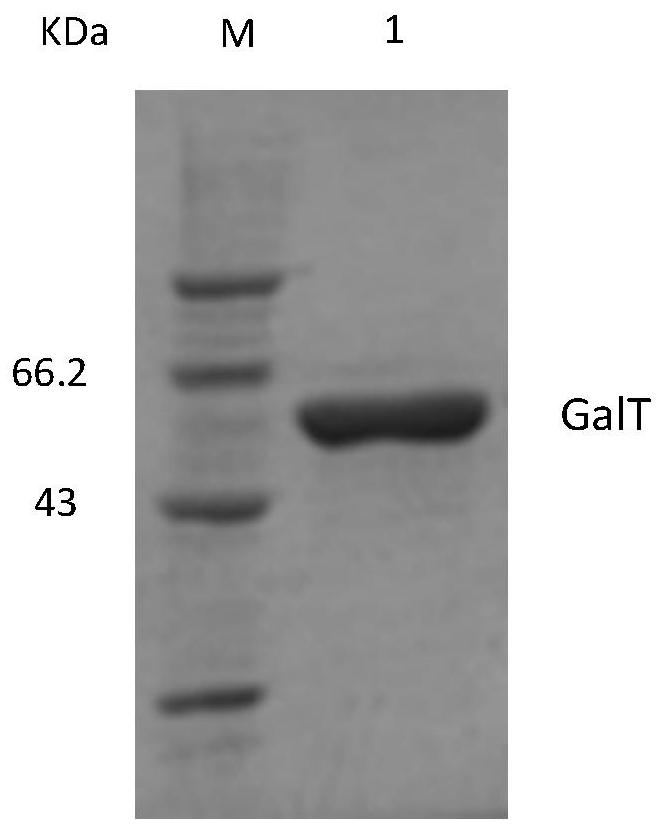
A method for expressing and preparing udp-glucose-hexose-1-phosphate uridine acyltransferase
A uridine phosphate and UDP-technology, which is applied in the field of genetic engineering, can solve the problems of unsuitability for large-scale preparation, low protein expression, cumbersome and time-consuming operations, etc., and achieves low cost, simple and fast cultivation, and high application. effect of value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
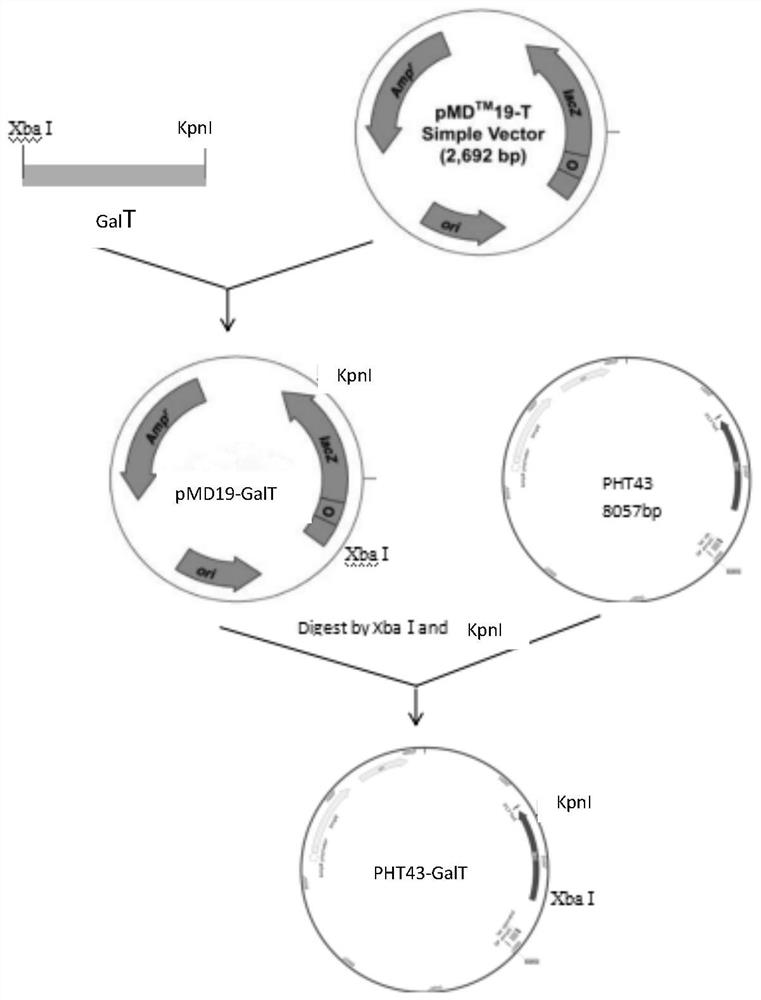
[0023] (1) Construction of recombinant expression vector pHT43-GalT
[0024] UDP-glucose-hexose-1-phosphate uridine acyltransferase (GalT) gene (sequence shown in SEQ ID NO: 1) is derived from Bifidobacterium longum JCM 1217, and after PCR amplification and purification, it is connected to the cloning vector pMD19, The recombinant plasmid pMD19-GalT was constructed.
[0025] The recombinant plasmid pMD19-GalT and the expression vector pHT43 were double-digested with XbaI and KpnI respectively, and ligated overnight at 16°C to obtain the recombinant expression vector pHT43-GalT.
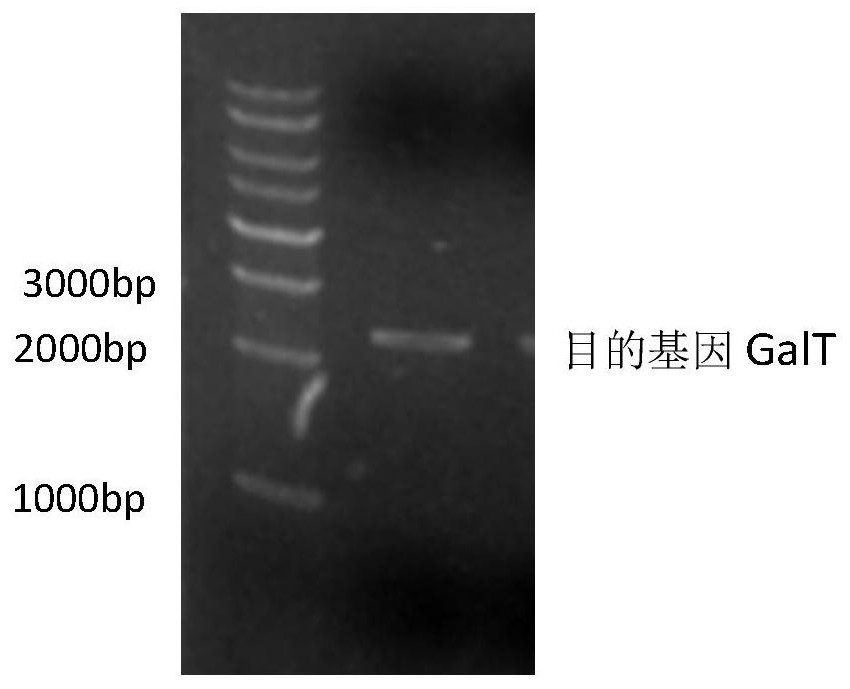
[0026] The recombinant expression vector pHT43-GalT was transformed into Bacillus subtilis WB800N, spread on LB plates containing chloramphenicol (5ug / mL) resistance, cultured overnight at 37°C, picked transformants, extracted recombinant plasmids and verified by double enzyme digestion. Such as figure 2 As shown, there are two fragments after digestion, the sizes are about 8000bp (expression vecto...
Embodiment 2
[0043] (1) Construction of recombinant expression vector pHT43-GalT
[0044] The recombinant plasmid pMD19-GalT prepared in Example 1 and the expression vector pHT43 were double digested with XbaI and KpnI respectively, and ligated overnight at 16°C to obtain the recombinant expression vector pHT43-GalT.
[0045] The recombinant expression vector pHT43-GalT was transformed into Bacillus subtilis WB800, spread on LB plate containing chloramphenicol (5ug / mL) resistance, cultured overnight at 37°C, picked transformants, extracted recombinant plasmids and verified by double enzyme digestion. Such as figure 2 As shown, there are two fragments after digestion, the sizes are about 8000bp (expression vector pHT43) and 2637bp (UDP-glucose-hexose-1-phosphate uridine acyltransferase), respectively, indicating that the connection is successful.
[0046] (2) Recombinant engineering bacteria
[0047] The constructed recombinant expression vector pHT43-GalT was transformed by electric sho...
Embodiment 3
[0053] (1) Construction of recombinant expression vector pMA5-GalT
[0054] The recombinant plasmid pMD19-GalT and the shuttle vector pMA5 were prepared according to Example 1, which were double-digested with XbaI and KpnI respectively, and ligated overnight at 16°C to obtain the recombinant expression vector pMA5-GalT.
[0055] The recombinant expression vector pMA5-GalT was transformed into Bacillus subtilis 168, coated with LB plates containing ampicillin (100ug / mL) resistance, cultured overnight at 37°C, the transformants were picked, and the recombinant plasmid was extracted and verified by double enzyme digestion. The build was successful.
[0056] (2) Recombinant engineering bacteria
[0057] The constructed recombinant expression vector pMA5-GalT was transformed by electric shock. Bacillus subtilis 168 electroporated competent cells were mixed with the recombinant expression vector pMA5-GalT plasmid, and then electric shock was performed after adding to the electric s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com