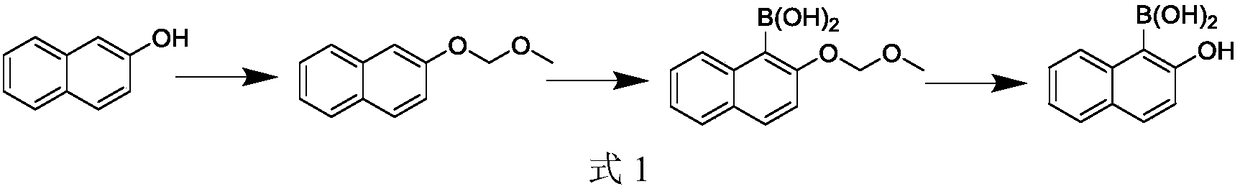
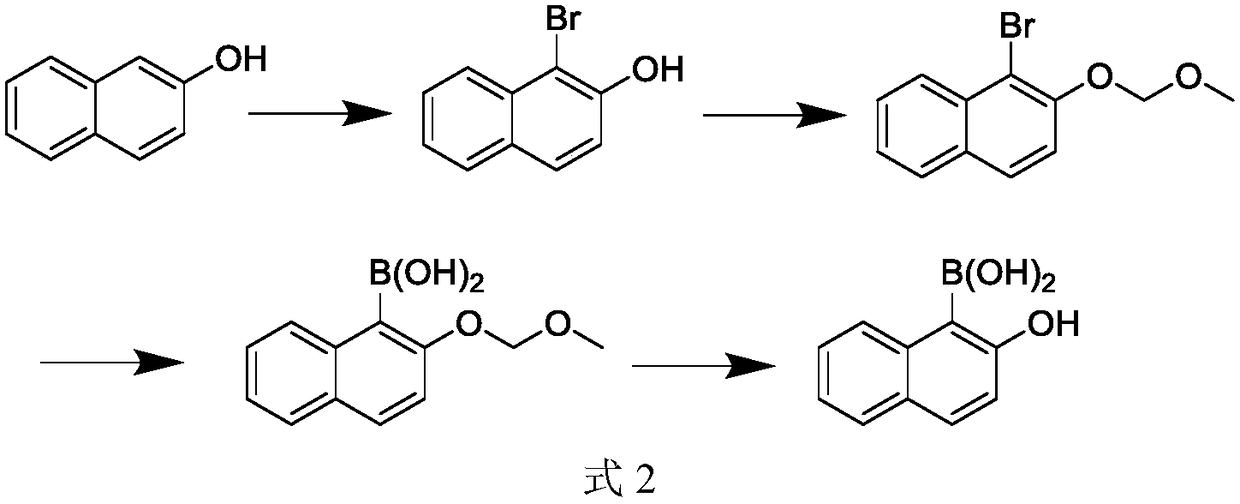
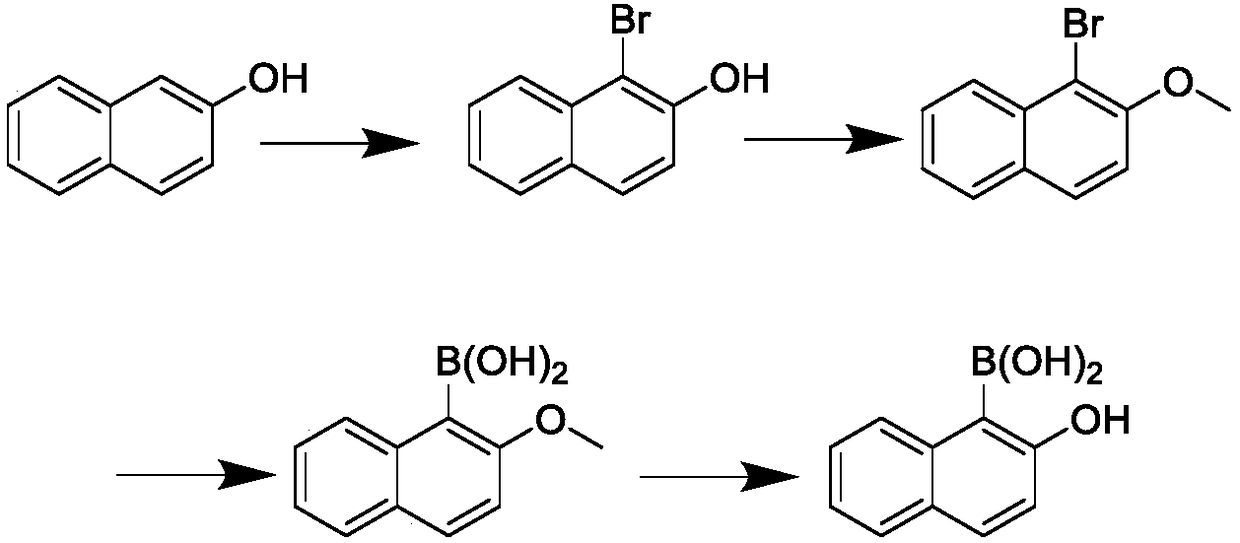
Synthesis method for 2-hydroxy naphthalene-1-boracic acid
A synthetic method and technology of hydroxynaphthalene, which is applied in the field of synthesis and purification of high-purity intermediate 2-hydroxynaphthalene-1-boronic acid, can solve problems such as unstable target products, achieve high yield, simple process steps, and easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, a kind of synthetic method of 2-hydroxynaphthalene-1-boronic acid, carries out following steps successively:
[0059] 1), the synthesis of 1-bromo-2-naphthol (using 2-naphthol and NBS to react synthetic 1-bromo-2-naphthol in solvent):
[0060]Add 144.2g of 2-naphthol (1mol) and 280ml of DMF (dimethylformamide) into a 1000ml reaction flask, stir evenly (stirring time is about 10 minutes), cool down to 0-5°C, and then add dropwise (dropwise Adding time is about 20 to 30 minutes) 178g NBS (N-bromosuccinimide, 1mol), using a water bath to control the temperature of the solution in the reaction bottle does not exceed 5 ° C, after the addition is completed, keep it warm at 0-5 ° C for 20 Minutes, adopt HPLC to monitor at this moment, raw material 2-naphthol is less than 1%.
[0061] Add 500ml of water to the liquid in the reaction bottle, quench (thereby the end of the reaction), then add 200ml of toluene, separate layers, the organic layer is located in the up...
Embodiment 3-1
[0075] Change the triisopropyl borate 1.8mol in the embodiment 1 step 3) into tributyl borate 1.9mol, and the rest are equal to the step 3) of the embodiment 1;
[0076] 118 g (0.58 mol) of off-white powder 2-methoxynaphthalene-1-boronic acid was obtained, with a content of 99.7% and a yield of 58%.
Embodiment 3-2
[0078] Change the triisopropyl borate 1.8mol in step 3) of embodiment 1 into trimethyl borate 2.0mol, and the rest are equal to step 3) of embodiment 1;
[0079] 119 g (0.59 mol) of off-white powder 2-methoxynaphthalene-1-boronic acid was obtained, with a content of 99.5% and a yield of 58.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com