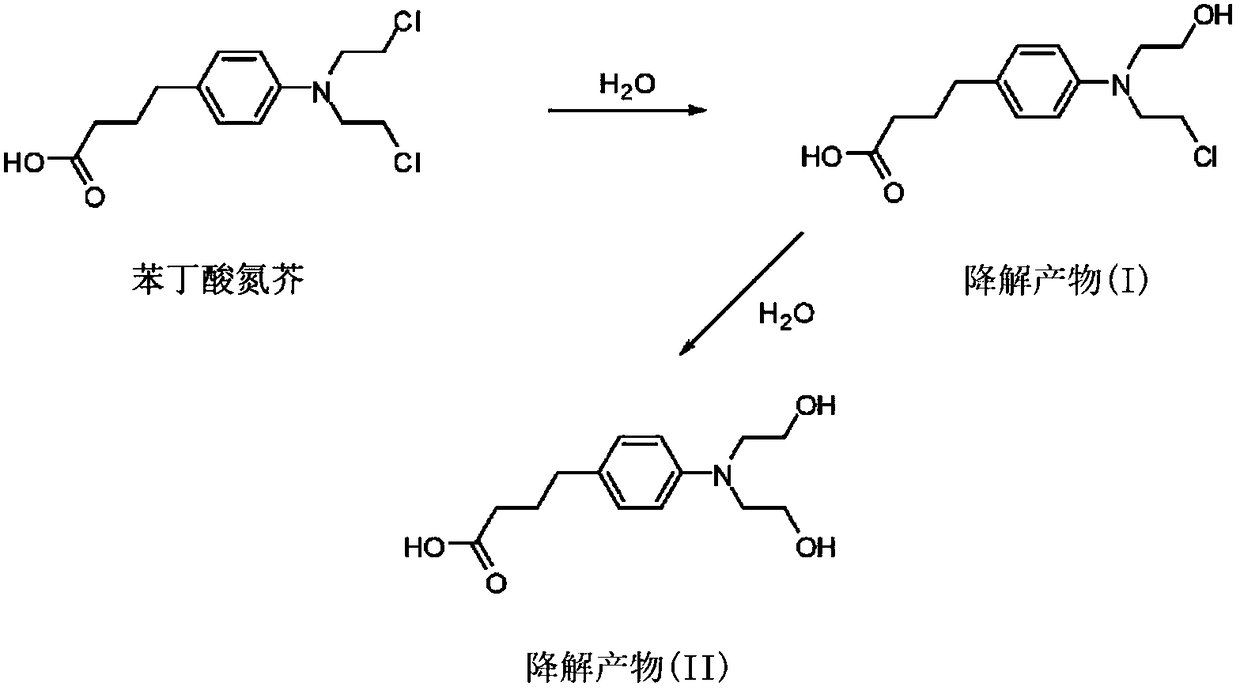
Formulations of chlorambucil
A technology for chlorambucil and preparations, applied in the field of compositions containing chlorambucil, can solve problems such as hindering research and development of chlorambucil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Example 1: Compositions containing chlorambucil and human serum albumin (HSA).
[0205] The weight ratio of the prepared chlorambucil to HSA is about 1:110.
[0206] Chlorambucil (4mg) was dissolved in methanol (0.5ml) in a vial to give a clear solution. Powdered HSA (440 mg) (natural fatty acid-free human serum albumin purchased from SeraCare Life Sciences, Cat. No. HS-455-80, which contains <0.2 mg / gm fatty acids) was dissolved In 8ml of water in a round bottom flask. At 0° C., the methanol solution of chlorambucil was slowly added dropwise to the HSA solution in the flask, and stirred. After the addition was complete, a clear solution was obtained. The resulting clear aqueous solution was lyophilized overnight to give a white solid.
[0207] A 100 mg lyophilized solid sample was reconstituted by adding 2 ml of water to give a clear aqueous solution.
Embodiment 2
[0208] Example 2: Composition comprising chlorambucil and human serum albumin (HSA).
[0209] The weight ratio of the prepared chlorambucil to HSA is about 1:80.
[0210] Chlorambucil (4mg) was dissolved in methanol (0.5ml) in a vial to give a clear solution. Powdered HSA (320 mg) (natural fatty acid-free human serum albumin purchased from SeraCare Life Sciences, Cat. No. HS-455-80, which contains <0.2 mg / gm fatty acids) was dissolved In 8ml of water in a round bottom flask. At 0° C., the methanol solution of chlorambucil was slowly added dropwise to the HSA solution in the flask, and stirred. After the addition was complete, a clear solution was obtained. The resulting clear aqueous solution was lyophilized overnight to give a white solid.
[0211] A 100 mg lyophilized solid sample was reconstituted by adding 2 ml of water to give a clear aqueous solution.
Embodiment 3
[0212]Example 3: Compositions containing chlorambucil and human serum albumin (HSA).
[0213] The weight ratio of the prepared chlorambucil to HSA is about 1:110.
[0214] Chlorambucil (4mg) was dissolved in methanol (0.5ml) in a vial to give a clear solution. HSA solution (440mg, 2.2ml) (purchased from Jeter Behring (CSL Behring) for infusion with 20% human serum albumin solution (product name: AlbuRx)) was added to 5.8ml of water in the round bottom flask, HSA solution (8ml) was obtained. At 0° C., the methanol solution of chlorambucil was slowly added dropwise to the HSA solution in the flask, and stirred. After the addition was complete, a clear solution was obtained. The resulting clear aqueous solution was lyophilized overnight to give a white solid.
[0215] A 100 mg lyophilized solid sample was reconstituted by adding 2 ml of water to give a clear aqueous solution. When visually observed after standing at room temperature for 3 hours, the clear aqueous solution r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com