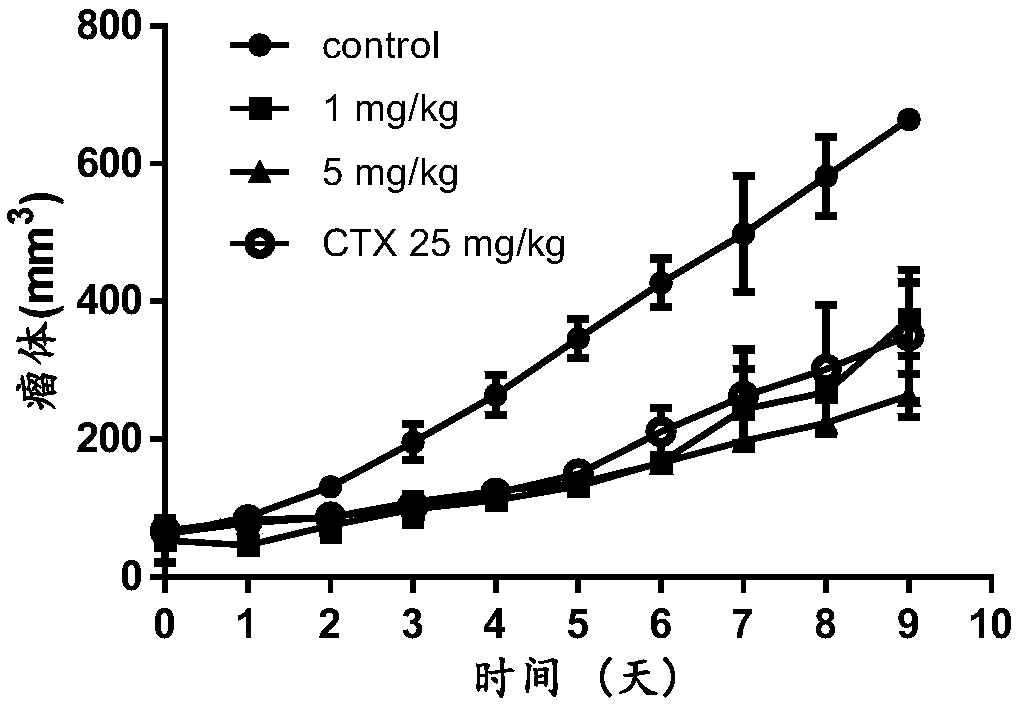
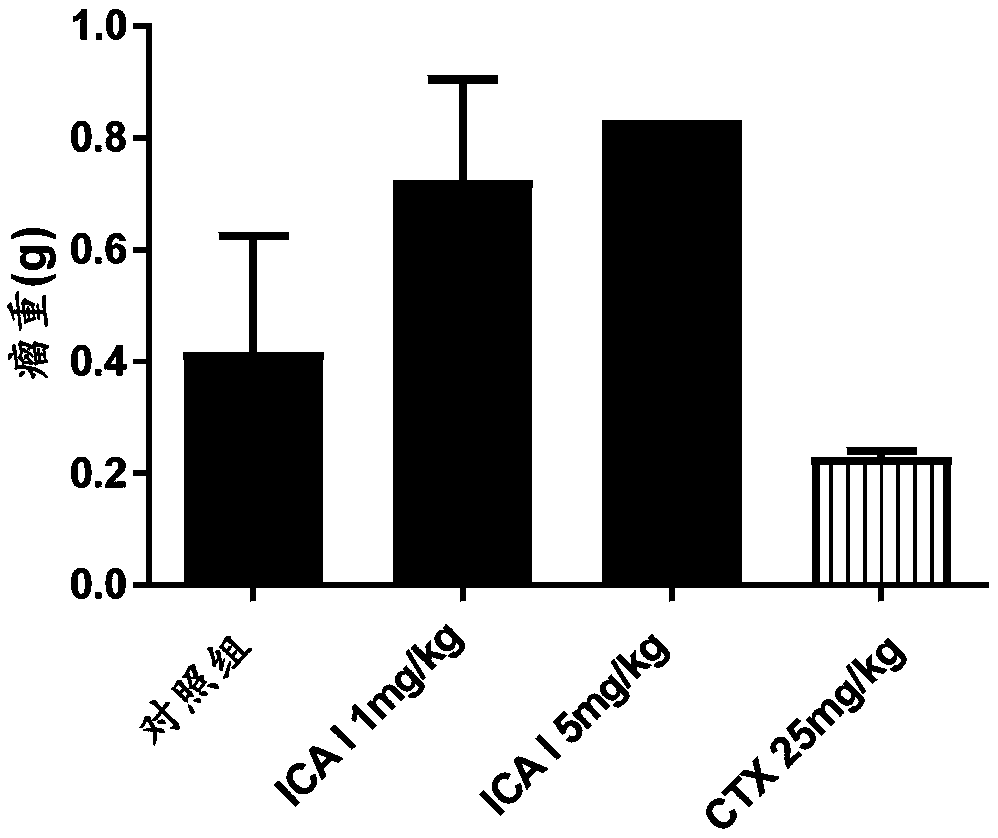
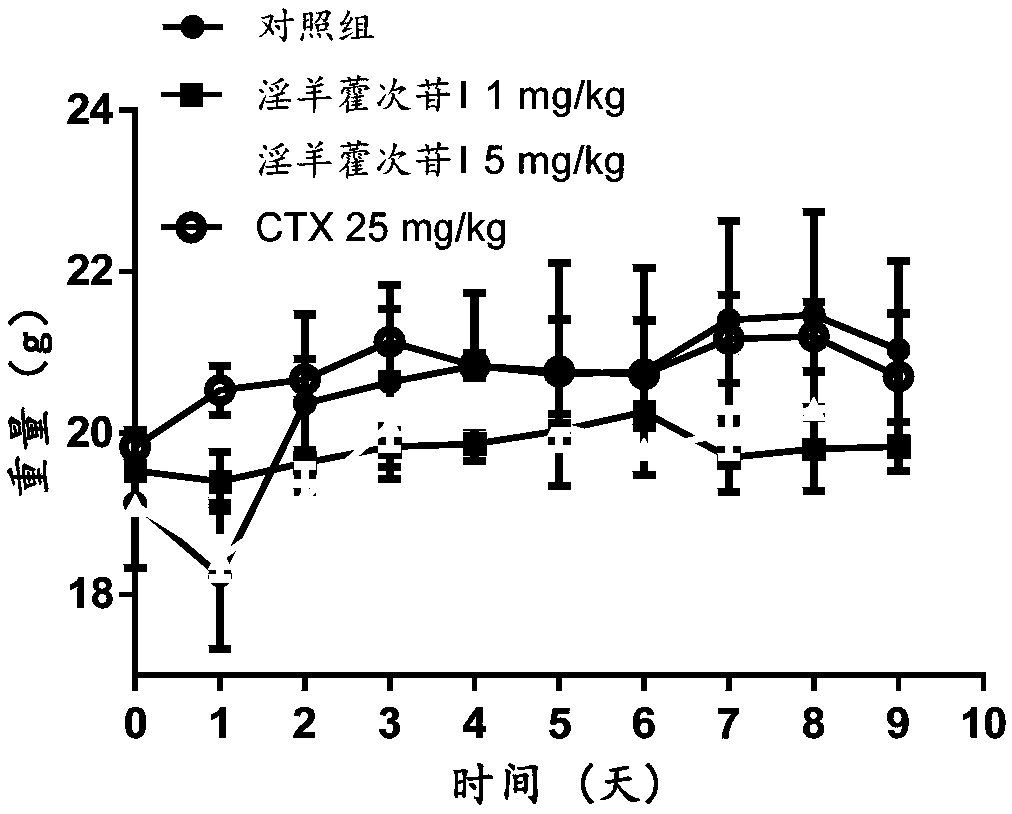
Icariside I compound, derivative, pharmaceutical salt and application
A technology of icariin and compound, applied in the directions of sugar derivatives, organic chemistry, drug combination, etc., can solve the problems of aggravation of the patient's condition, the economic burden of the patient, and the large dose.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: get 100mg icariside I, add 2mL of DMSO, 30mg iodomethane (CH 3 1), then add 10mg of silver oxide (Ag 2 O), stirred at 40 °C for 4 hours. The reaction solution was poured into distilled water, and then the precipitated yellow solid was suction-filtered, dried, and purified by column chromatography to obtain R 1 The group is the product of methoxy group.
Embodiment 2
[0034] Embodiment 2: get 100mg icariside I, add 2mL of DMSO, 100mg iodomethane (CH 3 1), then add 10mg of silver oxide (Ag 2O), stirred at 25 °C for 12 hours. The reaction solution was poured into distilled water, and then the precipitated yellow solid was suction-filtered, dried, and purified by column chromatography to obtain R 3 and R 1 The group is the product of methoxy group.
Embodiment 3
[0035] Example 3: Take 100 mg of icariside I, add 2 ml of DMSO, 50 mg of dimethyl sulfate, and stir at 80° C. for 2 hours. The reaction solution was poured into distilled water, and then the precipitated yellow solid was suction filtered, dried, and purified by column chromatography (silica gel column) to obtain R 3 The group is the product of methoxy group. Required R 3 Icaritin compounds with a methoxyl group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com