Synthesis method for 2, 4-dichloro-5-isopropoxyaniline
A technology of propoxyaniline and synthesis method, which is applied in two fields, can solve the problems such as difficult recovery of catalyst, difficult recovery of catalyst, easy poisoning of catalyst, etc., and achieve great economic benefits and industrial application prospects, easy control of reaction, and effects of not easy poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The invention discloses a synthesis method of 2,4-dichloro-5-isopropoxyaniline, specifically discloses a synthesis method of 2,4-dichloro-5-isopropoxyaniline, comprising the following steps:
[0021] S1. Putting auxiliary agents and catalysts into the reactor to obtain the first mixed solution;
[0022] S2. Putting an organic solvent and a raw material compound into the first mixed liquid, and feeding hydrogen gas into the reaction vessel, so that the original compound undergoes a reduction reaction with the hydrogen gas in the presence of an organic solvent, an auxiliary agent and a catalyst, And obtain the reaction product;
[0023] S3. Remove the catalyst and solvent from the reaction product to obtain 2,4-dichloro-5-isopropoxyaniline.
[0024] Wherein, the catalyst is a nano-catalyst, such as a nano-nickel catalyst.
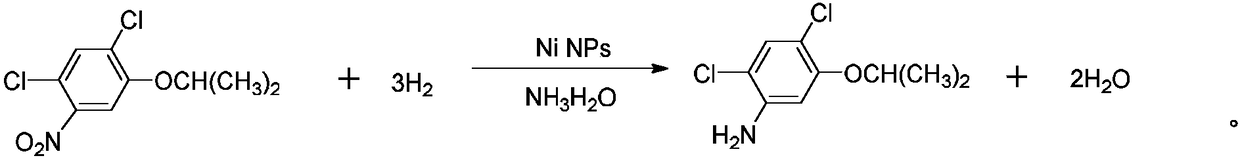
[0025] Overall chemical reaction equation of the present invention is as follows:
[0026]
Embodiment 1
[0028] The invention discloses a synthesis method of 2,4-dichloro-5-isopropoxyaniline, comprising the following steps:
[0029] S1. Add 4-6g of nano-catalyst with a particle size of 90-100nm and 48-53ml of adjuvant into a reaction kettle with a capacity of 1-2L, and start stirring to fully mix the two.
[0030] S2. After waiting for stirring for 20 to 30 minutes, add 392 to 400 ml of toluene and 247 to 251 g of 2,4-dichloro-5-isopropoxyaniline into the reaction kettle;
[0031] S3. Introduce nitrogen into the reactor to replace the air in the reactor, and then turn off the introduction of nitrogen after 8 to 10 minutes, and then inject hydrogen into the reactor to replace the nitrogen in the reactor , after another 8 to 10 minutes, turn off the introduction of hydrogen, and then feed nitrogen into the reaction kettle again to replace the hydrogen. After repeating three times, keep the hydrogen pressure in the reaction kettle at 2 to 3 MPa, raise the temperature to 98 to 104 ° ...
Embodiment 2
[0035] The invention discloses a synthesis method of 2,4-dichloro-5-isopropoxyaniline, comprising the following steps:
[0036] S1. Add 1.6-2.5g of nano-catalysts with a particle size of 10-20nm and 15-20ml of adjuvants into a reaction kettle with a capacity of 1-2L, and start stirring to fully mix the two. ;
[0037] S2. After waiting for stirring for 20 to 30 minutes, add 200 to 210 ml of dichloroethane and 247 to 251 g of 2,4-dichloro-5-isopropoxyaniline into the reaction kettle;
[0038] S3. Introduce nitrogen into the reactor to replace the air in the reactor, and then turn off the introduction of nitrogen after 8 to 10 minutes, and then inject hydrogen into the reactor to replace the nitrogen in the reactor , after another 8 to 10 minutes, turn off the introduction of hydrogen, and then feed nitrogen into the reactor again to replace the hydrogen. After repeating three times, keep the hydrogen pressure in the reactor at 4 to 5 MPa, raise the temperature to 75 to 85°C, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com