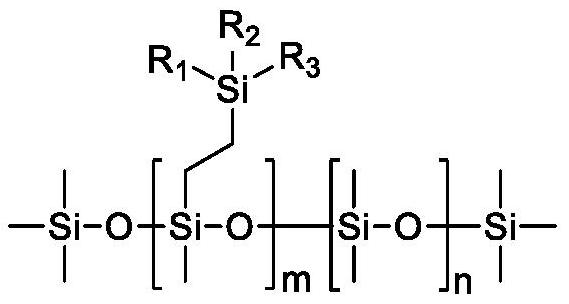
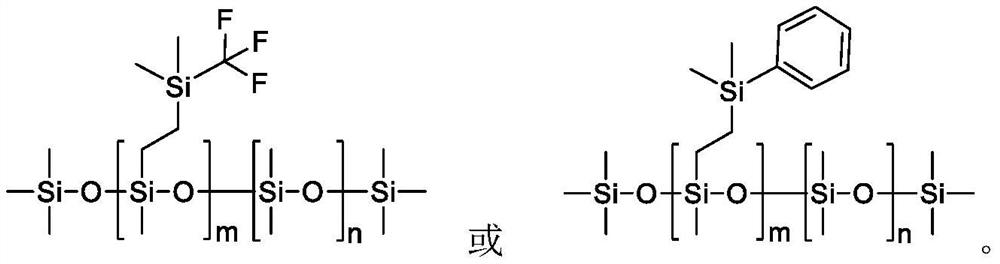
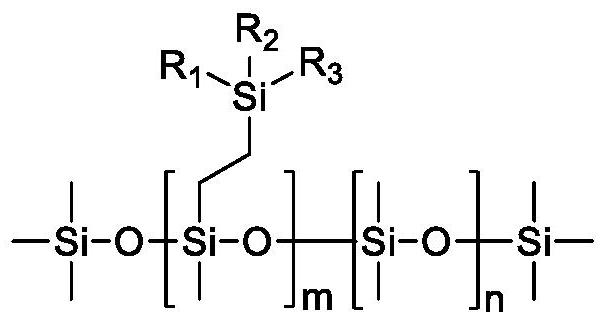
A kind of organosiloxane flame retardant and its preparation method and application
A technology of organic siloxane and flame retardant, which is applied in the field of electrochemistry, can solve the problems of decreased conductivity, large addition amount, and increased cost, and achieve the effects of low viscosity, small addition amount, and little influence on electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0038] Preparation Example 1: Preparation of Phenylsiloxane
[0039]Dimethylphenylvinylsilane is used as raw material, and it is mixed with octamethyldihydrotetrasiloxane, decamethyldihydropentasiloxane and dodecamethyldihydrohexasiloxane at a molar ratio of 1:1. Alkane and hexadecylmethyldihydrooctasiloxane were mixed, Karstedt catalyst was added, the temperature was raised to 80° C. under the protection of nitrogen, and the crude product was obtained after 4 hours of reaction. Then carry out rectification to remove unreacted raw materials, then add 10% activated carbon adsorption catalyst, stir at 70°C for 10 hours, then heat filter, remove activated carbon, and obtain pure phenylsiloxanes with different silicon contents. Phenylsiloxane obtained by reacting with octamethyldihydrotetrasiloxane, decamethyldihydropentasiloxane, dodecamethyldihydrohexasiloxane, hexadecylmethyldihydrooctasiloxane The alkane products are respectively marked as A, B, C, and D, and the calculated s...
preparation example 2
[0040] Preparation Example 2: Preparation of Fluorosiloxane
[0041] Vinyl (trifluoromethyl) dimethylsilane is used as raw material, and it is mixed with octamethyldihydrotetrasiloxane, decamethyldihydropentasiloxane and dodecamethyldihydrotetrasiloxane at a molar ratio of 1:1. Hydrogenhexasiloxane and hexadecylmethyldihydrooctasiloxane were mixed, Karstedt catalyst was added, the temperature was raised to 90°C under the protection of nitrogen, and the crude product was obtained after 4 hours of reaction. Then carry out rectification to remove unreacted raw materials, then add 10% activated carbon adsorption catalyst, stir at 70°C for 10 hours, then heat filter, remove activated carbon, and obtain pure fluorosiloxanes with different silicon contents. Phenylsiloxane obtained by reacting with octamethyldihydrotetrasiloxane, decamethyldihydropentasiloxane, dodecamethyldihydrohexasiloxane, hexadecylmethyldihydrooctasiloxane The alkane products are respectively marked as E, F, G, ...
Embodiment 1
[0046] in a glove box filled with argon (H 2 0<10ppm) to prepare 4 parts of electrolytic solution as described in Comparative Example 1, then add phenylsiloxane A, B, C, D to 4 parts of electrolytic solution respectively with a mass fraction of 1%, to obtain Electrolyte solutions 2A, 2B, 2C, 2D.
[0047] The prepared electrolytes 2A, 2B, 2C, and 2D were tested for SET and conductivity respectively, and the results are shown in Table 1; then 2A, 2B, 2C, and 2D were respectively injected into lithium cobaltate graphite batteries for normal temperature charge-discharge cycle test, The test results are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com