Apixaban related substance and preparation method and application thereof
A related substance, a technology for apixaban, applied in the field of apixaban related substances and their preparation, can solve problems such as difficult removal of impurities, and achieve the effects of mild reaction conditions, high purity and good controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
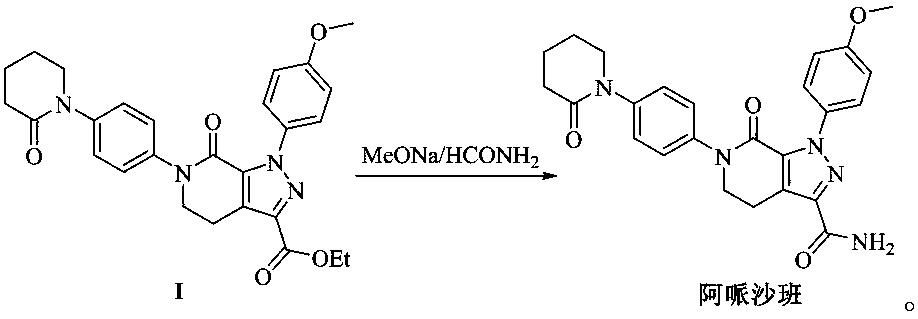
[0023] This embodiment provides a preparation method of related substance A of apixaban, comprising the following steps:
[0024] (1) In the first organic solvent, under basic conditions, compound II (chemical name: 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiper Pyridin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester) and 5-chloropentanoic acid ethyl ester A nucleophilic substitution reaction occurs at 0-100°C until the compound II disappears. After adding an appropriate amount of water to the reaction liquid to precipitate a solid, it is suction filtered, the filter cake is washed with water and isopropanol, and purified by column chromatography to obtain Intermediate III;
[0025] (2) Dissolve intermediate III in the second organic solvent, add strong alkali solution to the reaction solution under ice bath, adjust the pH of the reaction solution to 10-11, after the addition, adjust the temperature of the reaction solution to 0-40°C, hyd...
Embodiment 1
[0044] Embodiment 1 of the present invention provides a kind of preparation method of intermediate III, and its synthetic route is as follows:
[0045]
[0046] Specifically adopt the following method to prepare:
[0047] Compound II (2.0g, 4.9mmol), N,N-diisopropylethylamine (1.2mL, 7.4mmol), ethyl 5-chlorovalerate (0.97g, 5.9mmol) and N,N-dimethyl Add methyl formamide (12mL) into the reaction bottle and mix evenly, react the reaction solution at 80°C for 7 hours, detect the reaction by TLC until compound II disappears, add water (12mL) to the reaction solution, after the solid precipitates, continue to stir for 0.5 hours , suction filtration, and the filter cake was washed with water and isopropanol to obtain a crude product, which was purified by column chromatography to obtain a light yellow solid, namely compound III.
[0048] The light yellow solid obtained by this method was 1.8 g, and the yield was 68%.
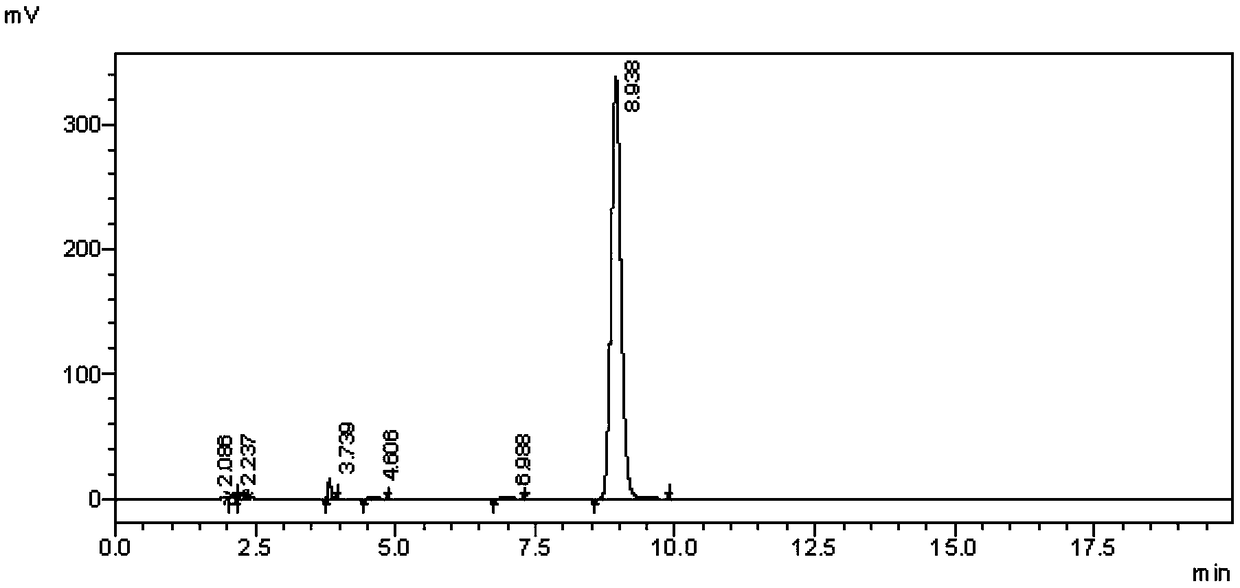
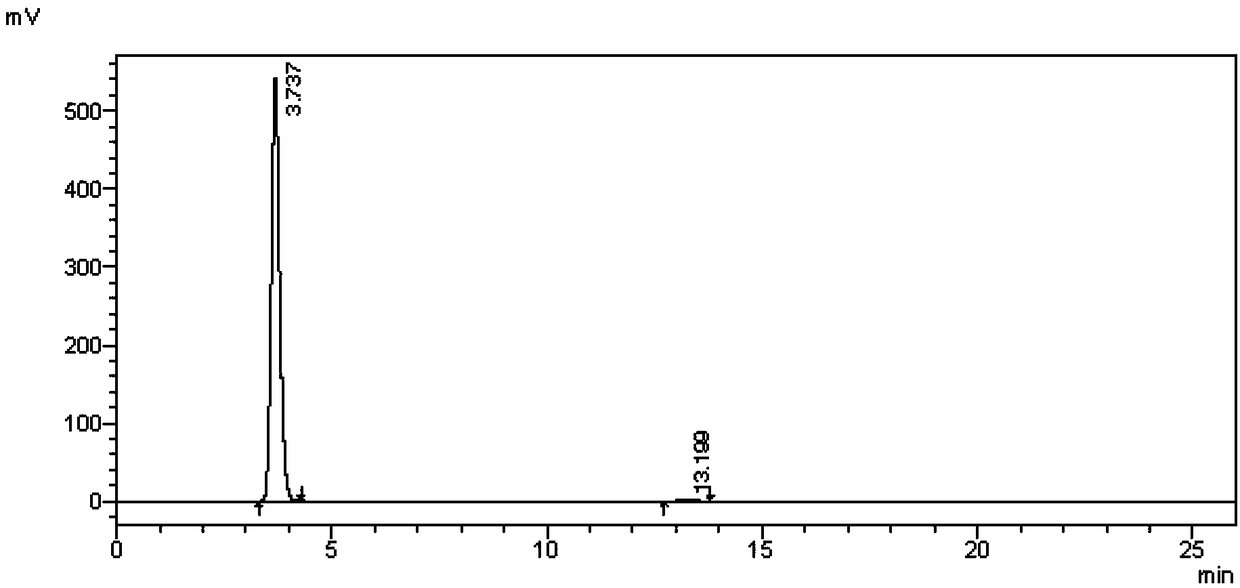
[0049] The intermediate III prepared in the present embodim...
Embodiment 2
[0053] Embodiment 2 of the present invention provides a method for preparing intermediate III, which is specifically prepared by the following method:
[0054] Compound II (1.3 g, 3.2 mmol), triethylamine (0.7 mL, 4.8 mmol), ethyl 5-chlorovalerate (0.63 g, 3.8 mmol) and N,N-dimethylformamide (8 mL) were added Mix well in the reaction flask, react the reaction solution at 80°C for 7 hours, detect the reaction by TLC until compound II disappears, add water (8mL) to the reaction solution, after the solid precipitates, continue to stir for 0.5 hours, filter with suction, and filter the cake with water Wash with isopropanol to obtain a crude product, which is purified by column chromatography to obtain a light yellow solid, namely compound III.
[0055] The light yellow solid obtained by this method was 1.1 g, and the yield was 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com