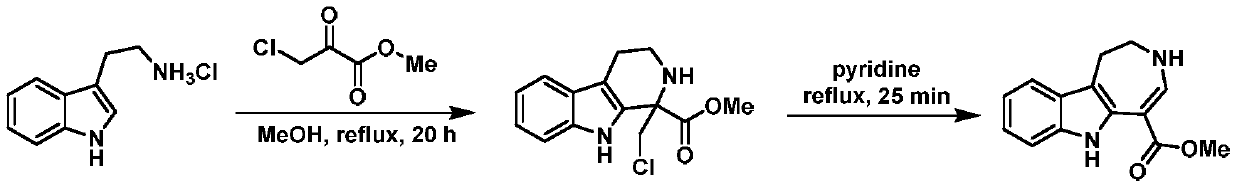
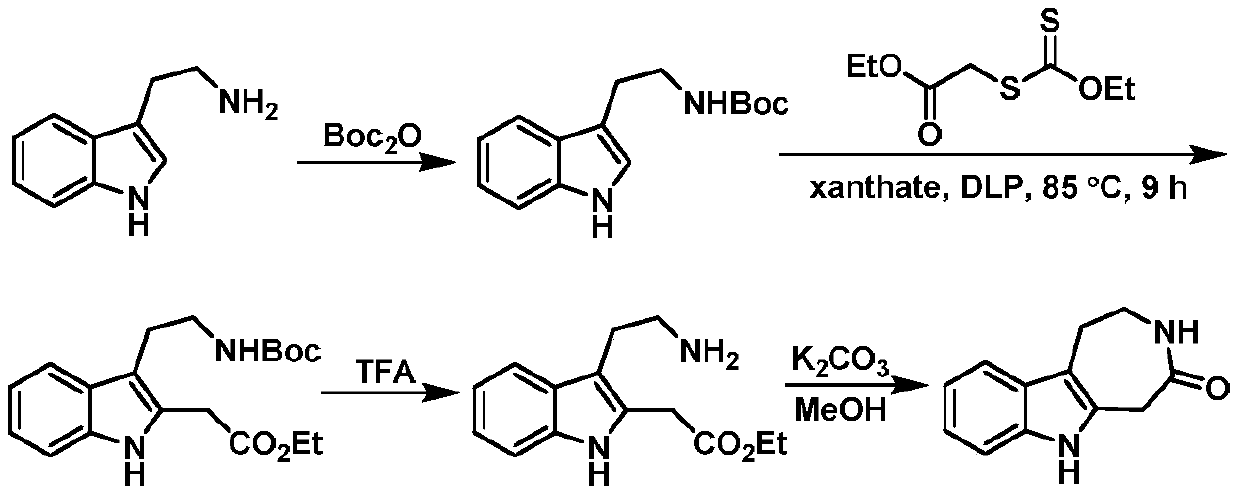
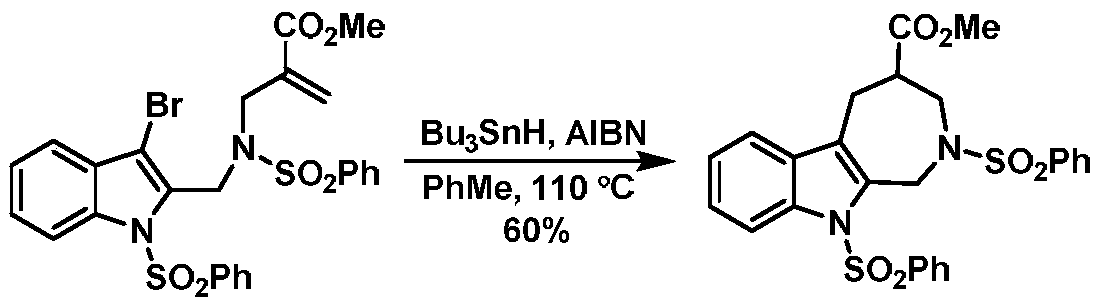
A kind of synthesis method of indoloazepine seven-membered ring catalyzed by monovalent silver
A synthesis method and seven-membered ring technology are applied in the field of synthesis of indoloazepine seven-membered rings, can solve the problems of poor atom economy, expensive reagents, complicated substrate preparation and the like, and achieve low price and reaction time. Short, simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add substrate a1 (0.1mmol, 43mg) into a 25mL eggplant-shaped flask, add 5mL of toluene solution, then add 1mL of toluene solution of silver trifluoromethanesulfonate (0.01mmol, 2.6mg), stir at room temperature, and stir for 6h , after the completion of the reaction, the target product b1 was separated by flash column chromatography (n-hexane:ethyl acetate=10:1) with a yield of 80%.
[0043] The reaction formula of Example 1 is:
[0044]
[0045] The spectral data of product b1 is: ESI-MS (m / z): 451[M+Na] + ; 1 H-NMR (600MHz, DMSO) δ7.74 (d, J = 8.2Hz, 2H), 7.51 (d, J = 7.9Hz, 1H), 7.34–7.44 (m, 5H), 7.24–7.28 (m, 3H ),7.12(t,J=7.5Hz,1H),7.03(t,J=7.5Hz,1H),7.00(s,1H),3.85–3.88(m,2H),2.93–2.95(m,5H) ,2.35(s,3H).
Embodiment 2
[0047] Add substrate a2 (0.1mmol, 45mg) into a 25mL eggplant-shaped flask, add 5mL of toluene solution, then add 1mL of silver trifluoromethanesulfonate (0.01mmol, 2.6mg) in toluene mixed solution, stir at room temperature, and stir the reaction 5h, after the reaction was completed, the target product b2 was separated by flash column chromatography (n-hexane:ethyl acetate=10:1) with a yield of 85%.
[0048] The reaction formula of Example 2 is:
[0049]
[0050] The spectral data of product b2 is: ESI-MS (m / z): 469[M+Na] + ; 1 H-NMR (600MHz, DMSO) δ7.74 (d, J = 8.2Hz, 2H), 7.51 (d, J = 7.9Hz, 1H), 7.39 (d, J = 8.2Hz, 2H), 7.23–7.30 ( m,5H),7.11–7.15(m,1H),7.01–7.05(m,1H),6.98(s,1H),3.85–3.87(m,2H),2.96(s,3H),2.92–2.94( m,2H), 2.36(s,3H).
Embodiment 3
[0052]Add substrate a3 (0.1mmol, 44mg) into a 25mL eggplant-shaped flask, add 5mL of toluene solution, then add 1mL of toluene solution of silver trifluoromethanesulfonate (0.01mmol, 2.6mg), stir at room temperature, and stir for 4h , after the reaction was completed, the target product b3 was separated by flash column chromatography (n-hexane:ethyl acetate=5:1) with a yield of 81%.
[0053] The reaction formula of example 3 is:
[0054]
[0055] The spectral data of product b3 is: ESI-MS (m / z): 457[M+Na] + ; 1 H-NMR (600MHz, DMSO) δ7.72 (d, J = 8.2Hz, 2H), 7.56 (dd, J = 5.2, 1.1Hz, 1H), 7.51 (d, J = 8.0Hz, 1H), 7.40 ( d,J=8.2Hz,2H),7.32(d,J=8.0Hz,1H),7.13–7.16(m,2H),7.10(dd,J=5.2,3.5Hz,1H),7.01–7.06(m ,2H), 3.86–3.89(m,2H), 3.12(s,3H), 2.88–2.92(m,2H), 2.36(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com