Valsartan amlodipine compound tablet and preparation method thereof
A technology of compound tablet and amlodipine besylate, which is applied in the field of medicine, can solve the problems such as the decrease of dissolution rate, and achieve the effects of stable dissolution rate, reduced rate of decline and ensuring effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
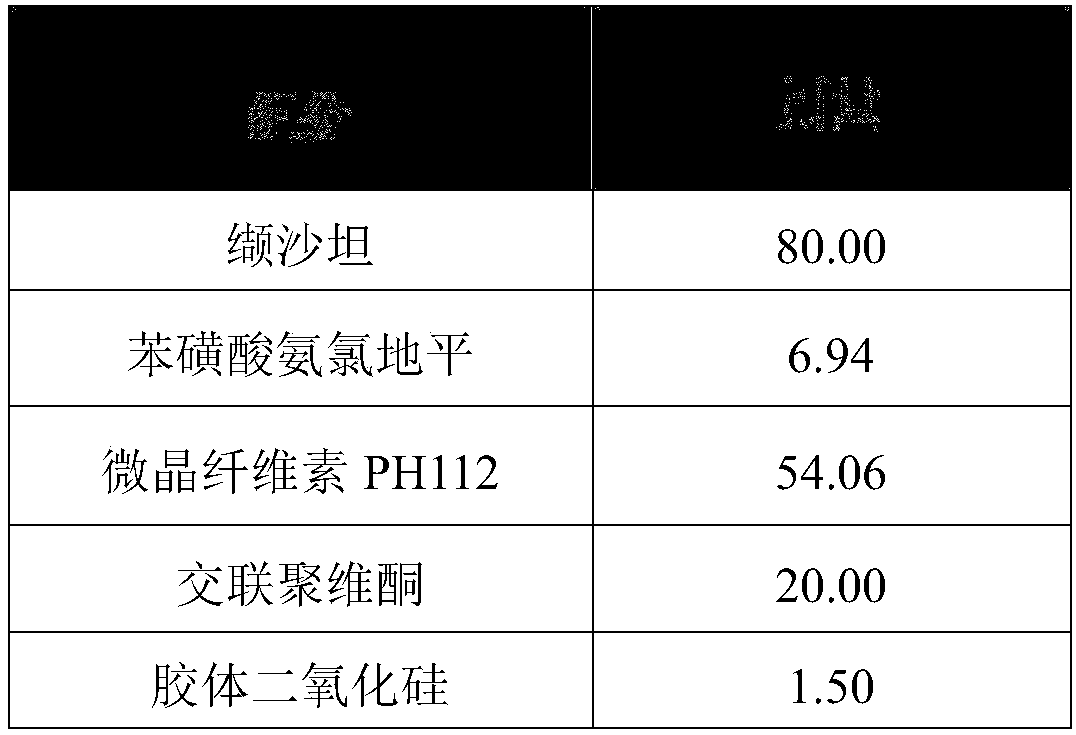
[0034] Each component in the valsartan amlodipine compound tablet described in the present embodiment and consumption thereof are shown in the following table:
[0035]
[0036]
[0037] The preparation method is as follows: mix the valsartan, amlodipine besylate, microcrystalline cellulose PH 112, cross-linked povidone and colloidal silicon dioxide in a mixer, and granulate through a dry granulator. Finally, add the magnesium stearate of formula quantity again, after mixing, compressing tablet both obtains.
Embodiment 2
[0039] Each component in the valsartan amlodipine compound tablet described in the present embodiment and consumption thereof are shown in the following table:
[0040] components
[0041] The preparation method is as follows: mixing valsartan, amlodipine besylate, microcrystalline cellulose PH 112, microcrystalline cellulose PH113, crospovidone and colloidal silicon dioxide in a mixer, After being granulated by a dry granulator, the formula amount of magnesium stearate is added, mixed evenly, and then compressed into tablets.
Embodiment 3
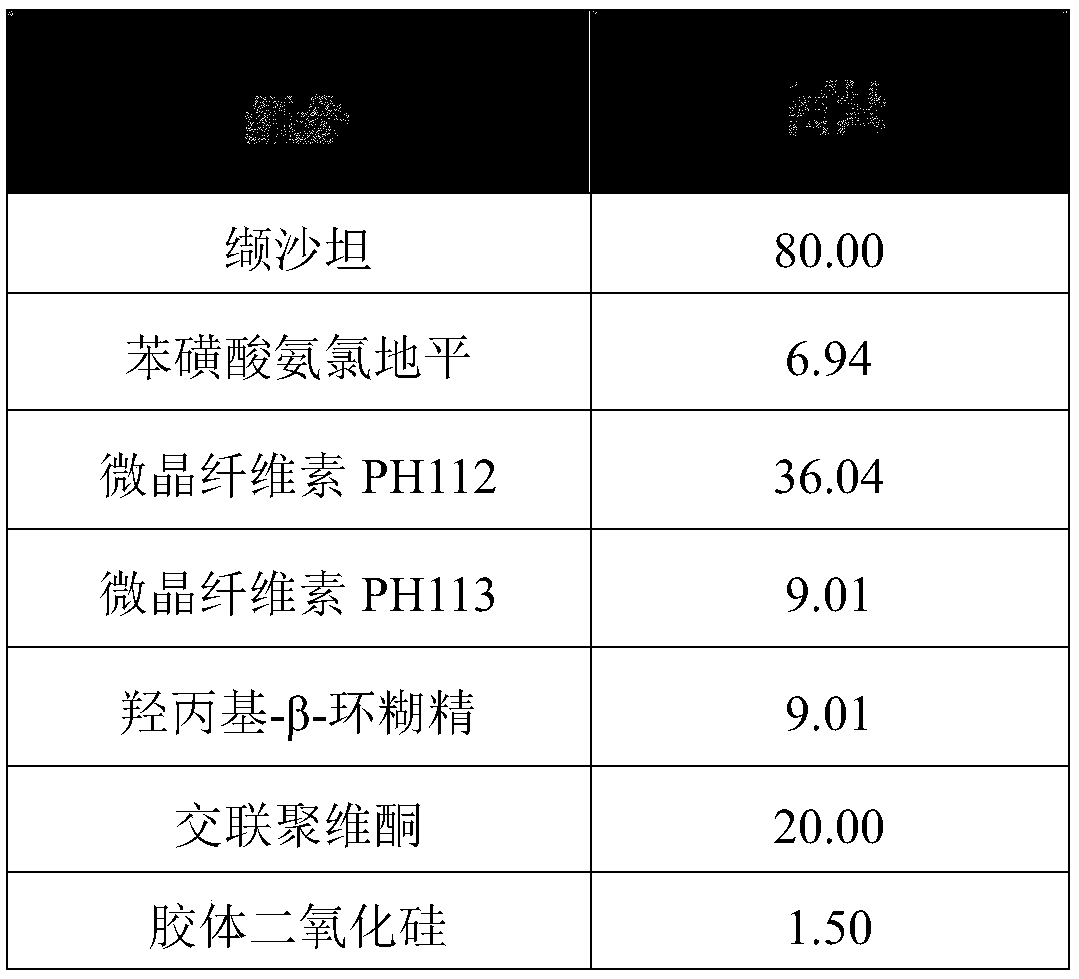
[0043] Each component in the valsartan amlodipine compound tablet described in the present embodiment and consumption thereof are shown in the following table:
[0044] components
[0045] The preparation method is as follows: valsartan, amlodipine besylate, microcrystalline cellulose PH 112, microcrystalline cellulose PH 113, hydroxypropyl-β-cyclodextrin, crospovidone and The colloidal silicon dioxide is mixed evenly in a mixer, and after being granulated by a dry granulator, the prescribed amount of magnesium stearate is added, mixed evenly, and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com