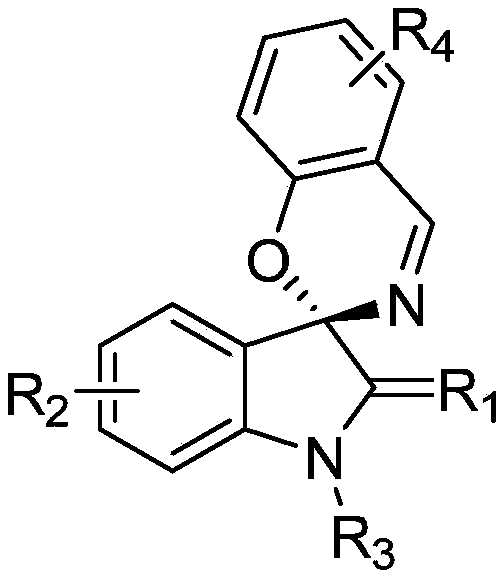
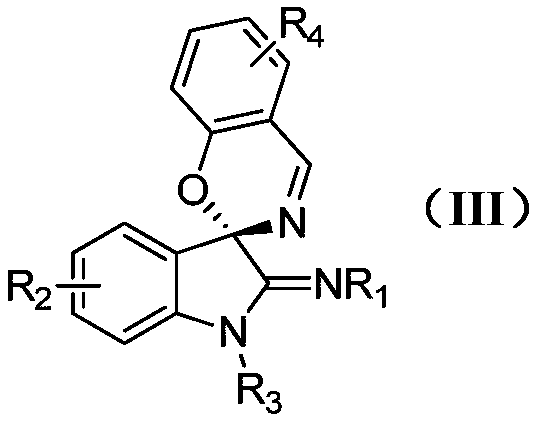
Indole-spirooxazine heterocyclic compound and preparation method thereof
A compound, the technology of indole spiro, is applied in the field of indole skeleton derivatives, which can solve problems such as uncommon methods, and achieve the effects of good greenness, high chemoselectivity, and wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
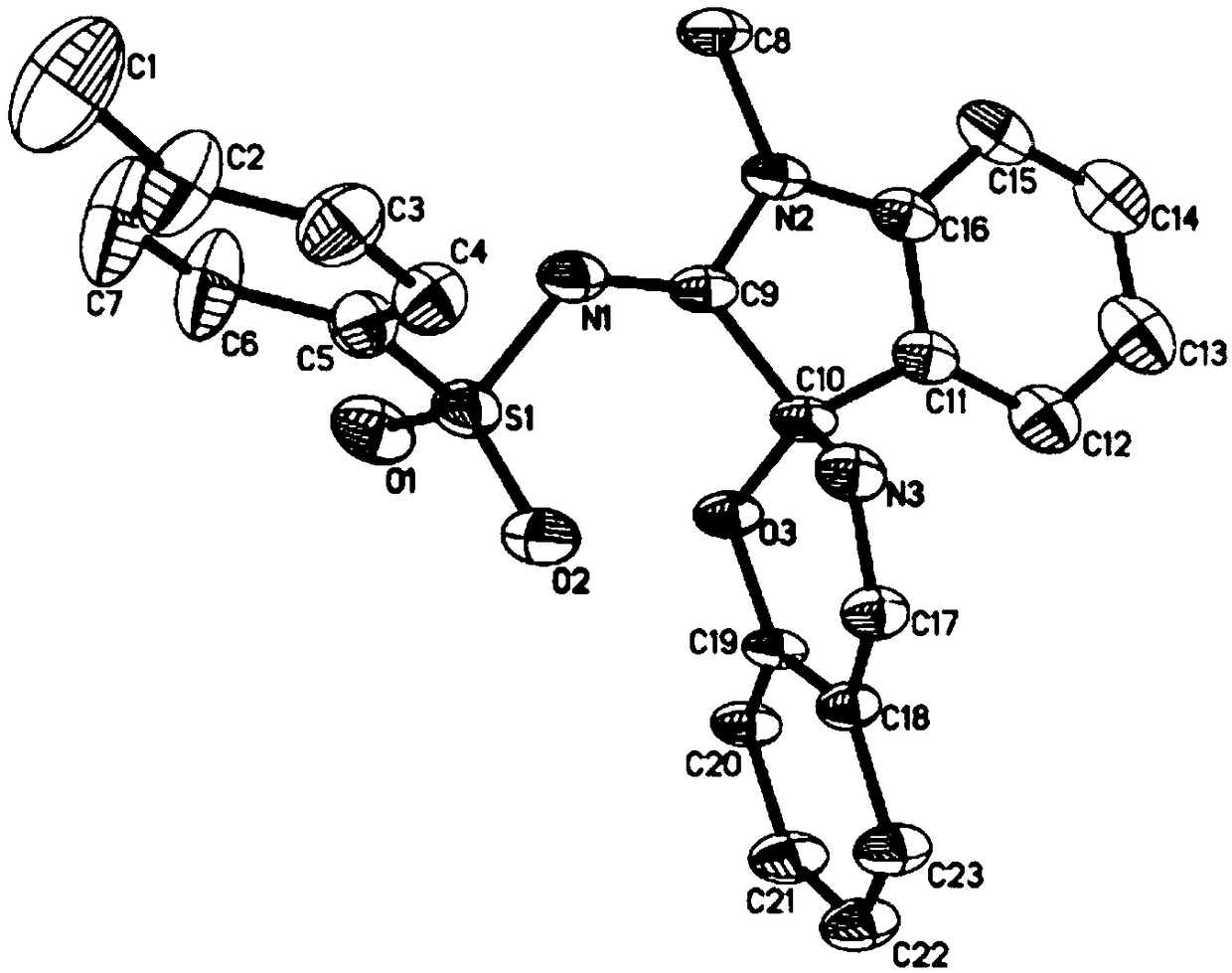
[0026] In a 25mL Schlenk reaction tube equipped with a magnetic stir bar, add the catalyst Rh 2 (esp) 2 (0.01mmol, 0.02equiv.), N-(3-diazo-1-methylindoline-2-ylidene)-4-methylbenzenesulfonamide (1.5mmol, 3.0equiv), benzisoxazole (0.5mmol, 1.0equiv.) and dichloromethane (5mL), nitrogen protection. The reaction solution was placed in an oil bath at 60° C. for about 2 to 4 hours. TLC detected that the reaction was complete, and then the reaction solution was cooled to room temperature. During the aftertreatment, the catalyst is removed by suction filtration through a sand core funnel equipped with silica gel, and the resulting filtrate is separated by flash column chromatography to obtain pure product 4-methyl-N-(1'-methylspiro[benzo[e] [1,3]oxazine-2,3'-indoline]-2'-methylene)benzenesulfonamide. Yield: 74%. The following is the NMR experimental data of product 3a:
[0027] 1H NMR (400MHz, DMSO) δ: 8.50(s, 1H), 7.69(d, J=8.2Hz, 2H), 7.53(d, J=6.6Hz, 1H), 7.48(t,...
Embodiment 2
[0030]
[0031] In a 25mL Schlenk reaction tube equipped with a magnetic stir bar, add the catalyst Rh 2 (esp) 2 (0.01mmol, 0.02equiv.), N-(3-diazo-1-methylindoline-2-ylidene)-4-methylbenzenesulfonamide (1.5mmol, 3.0equiv.), 5-methyl phenylisoxazole (0.5mmol, 1.0equiv.) and dichloromethane (5mL), nitrogen protection. The reaction solution was placed in an oil bath at 60° C. for about 2 to 4 hours. TLC detected that the reaction was complete, and then the reaction solution was cooled to room temperature. During the post-treatment, the catalyst is removed by suction filtration through a sand core funnel equipped with silica gel, and the obtained filtrate is separated by flash column chromatography to obtain the pure product N-(1',6-dimethylspiro[benzo[e][1 ,3]oxazine-2,3'-indoline]-2'-methylene)-4-methylbenzenesulfonamide. Yield: 58%. The following is the NMR experimental data of product 3b:
[0032] 1 H NMR (400MHz, DMSO) δ: 8.44(s, 1H), 7.69(d, J=8.2Hz, 2H), 7.46(t, J...
Embodiment 3
[0035]
[0036] In a 25mL Schlenk reaction tube equipped with a magnetic stir bar, add the catalyst Rh 2 (esp) 2 (0.01mmol, 0.02equiv.), N-(3-diazo-1-methylindoline-2-ylidene)-4-methylbenzenesulfonamide (1.5mmol, 3.0equiv), 5-chloro Benzisoxazole (0.5mmol, 1.0equiv.) and dichloromethane (5mL), nitrogen protection. The reaction solution was placed in an oil bath at 60° C. for about 2 to 4 hours. TLC detected that the reaction was complete, and then the reaction solution was cooled to room temperature. During the aftertreatment, the catalyst is removed by suction filtration through a sand core funnel equipped with silica gel, and the gained filtrate is separated by flash column chromatography to obtain pure product N-(6-chloro-1'-methylspiro[benzo[e][ 1,3]oxazine-2,3'-indoline]-2'-methylene)-4-methylbenzenesulfonamide. Yield: 52%. The following is the NMR experimental data of product 3c:
[0037] 1 H NMR (400MHz, DMSO) δ: 8.49(s, 1H), 7.72–7.66(m, 3H), 7.53–7.47(m, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com