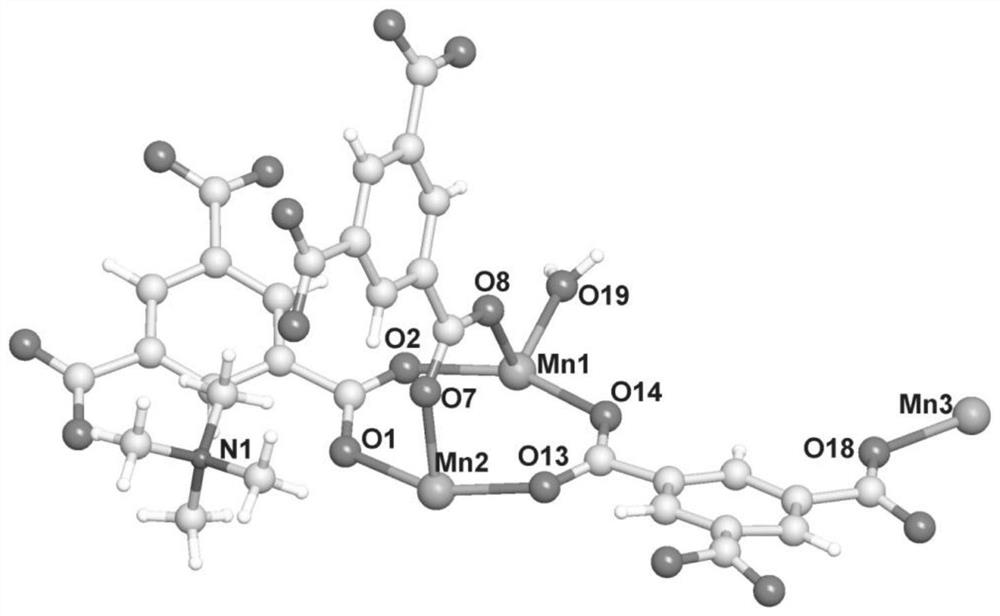
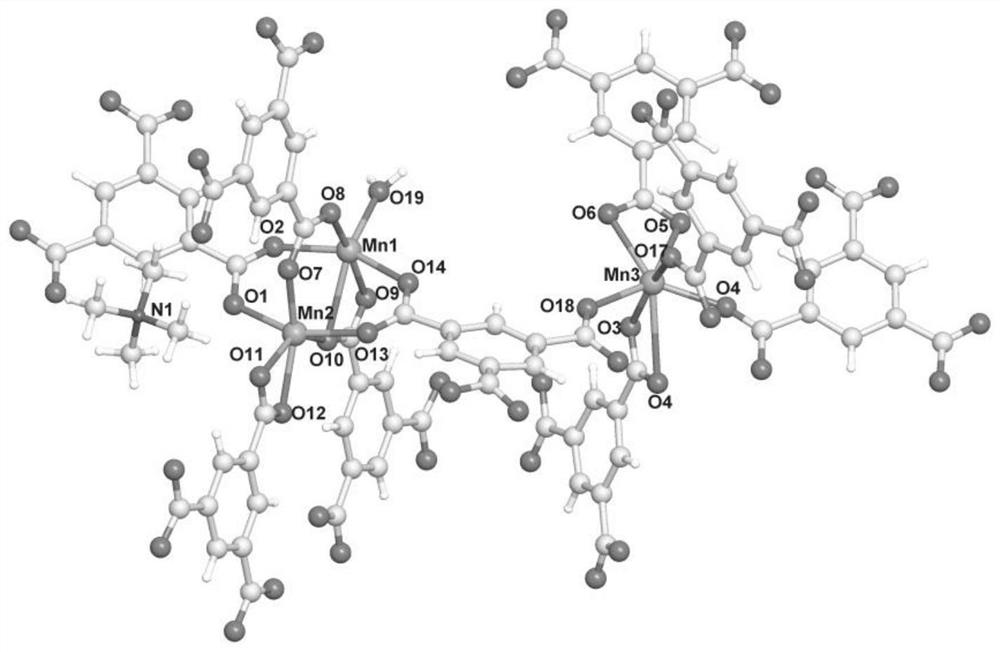
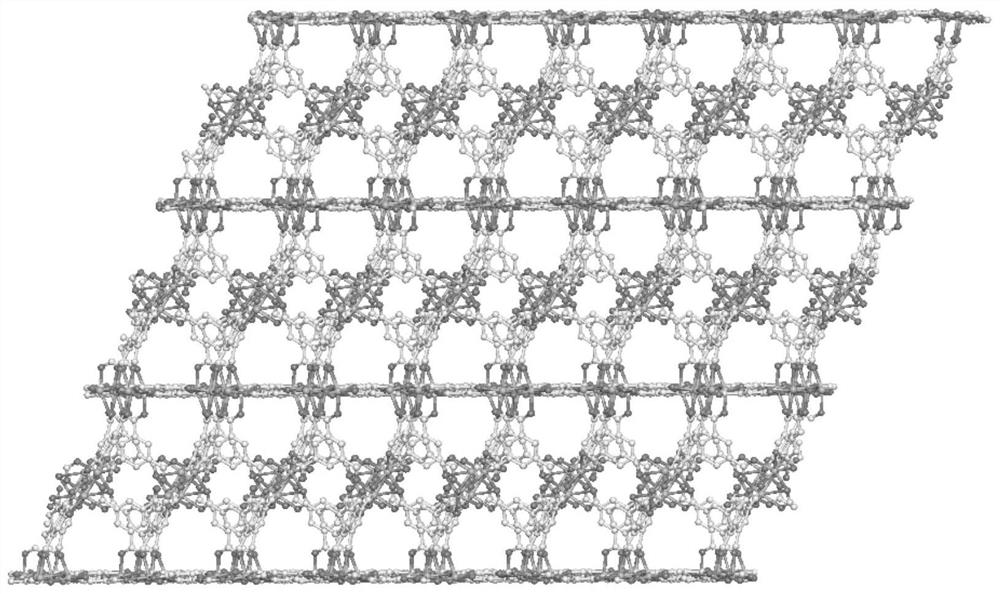
A kind of manganese coordination polymer and its preparation method and application
A coordination polymer, coordination technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of manganese coordination polymer, comprising the following steps:
[0038] 1) Dissolve 0.1 mmol of trimesic acid in 15 mL of N,N-dimethylacetamide solution, and transfer the solution into a reaction kettle to prepare a mixed solution;
[0039] 2) Take 0.1 mmol of manganese acetate and add it to the mixed solution of step 1), and drop glycolic acid into the mixed solution, stir while dripping, and adjust the pH value to 6;
[0040] 3) Seal the reaction kettle, keep the temperature at 120°C for 72 hours, then slowly cool to room temperature, the filtrate is yellow and clear, filter and wash the precipitate with fresh DMA solvent, pick out the colorless flaky hexagonal crystals under the microscope, which is the manganese complex. bit polymer. The DMA solvent is N,N-dimethylacetamide.
Embodiment 2
[0042] A preparation method of manganese coordination polymer, comprising the following steps:
[0043]1) Dissolve 0.2 mmol of trimesic acid in 15 mL of N,N-dimethylacetamide solution, and transfer the solution into a reaction kettle to prepare a mixed solution;
[0044] 2) Add 0.2 mmol of manganese acetate into the mixed solution of step 1), and drop glycolic acid into the mixed solution, stir while dripping, and adjust the pH value to 6;
[0045] 3) Seal the reaction kettle, keep the temperature at 140°C for 72 hours, then slowly cool to room temperature, the filtrate is yellow and clear, filter and wash the precipitate with fresh DMA solvent, pick out the colorless flaky hexagonal crystals under the microscope, which is the manganese complex. bit polymer. The DMA solvent is N,N-dimethylacetamide.
[0046] Examples 1-2 use trimesic acid as a ligand and manganese acetate to obtain manganese coordination polymers by solvothermal method. The appearance is colorless and transp...
Embodiment 3
[0048] A preparation method of manganese coordination polymer, comprising the following steps:
[0049] 1) Dissolve 0.2 mmol of trimesic acid in 15 mL of N,N-dimethylformamide solution, and transfer the solution into a glass bottle to prepare a mixed solution;
[0050] 2) Add 0.2 mmol of manganese chloride to the mixed solution of step 1), and drop glycolic acid into the mixed solution, stir while dripping, and adjust the pH value to 7;
[0051] 3) Seal the reaction kettle, keep the temperature at 140°C for 48 hours, then slowly cool to room temperature, filter, and wash the precipitate with fresh DMF solvent, pick out the colorless hexagonal flaky crystals under the microscope, which is the manganese coordination polymer . DMF is N,N-dimethylformamide.
[0052] This example uses trimesic acid as a ligand and manganese chloride to obtain a manganese coordination polymer through a solvothermal method. The appearance is colorless and transparent flake hexagonal crystals, taste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com