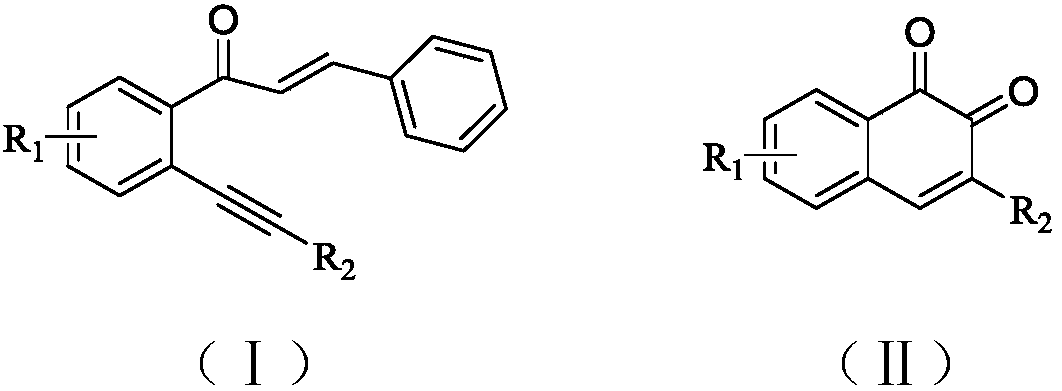
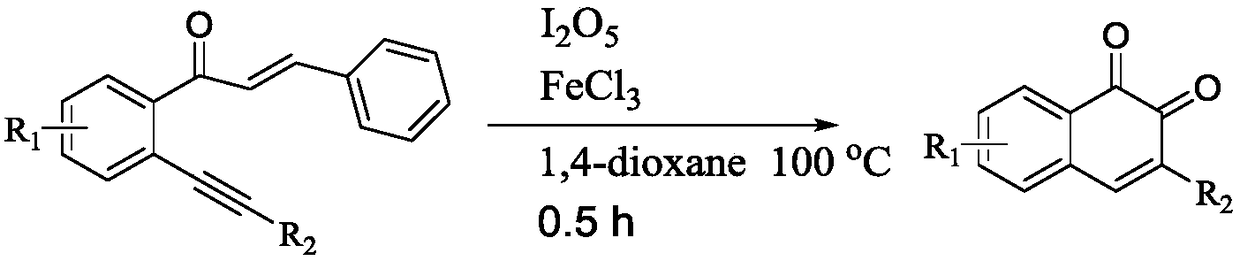Method for synthesizing 1,2-naphthoquinone and derivatives thereof
A synthesis method and derivative technology, applied in the preparation of oxidized quinone, organic chemistry, etc., can solve problems such as complex processes, and achieve the effects of short reaction time, high reaction efficiency, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
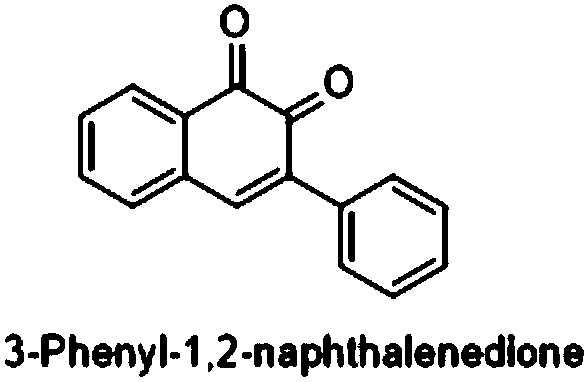
[0031] Add 0.3mmol (E)-3-phenyl-1-(2-(phenylethynyl)phenyl)prop-2-en-1-one, 0.03mmol ferric chloride, 0.6mmol diiodine pentoxide to In a 15mL thick-walled pressure-resistant reaction tube, add 3mL of 1,4-dioxane as a solvent. Next, magnetic stirring was performed at 100° C. for 0.5 hours. After cooling to room temperature, add two tablespoons of column chromatography silica gel (100-200 mesh) to the reaction solution, and remove the solvent by distillation under reduced pressure, and then separate the product 3-phenyl-1,2-naphthoquinone by column chromatography Pure product (petroleum ether / ethyl acetate = 10:1 as eluent). The material was a red solid in 57% yield.
[0032] Characterization data: 1 H NMR (500MHz, CDCl 3 ):δ7.31–7.47(m,8H),7.58(m,1H,),8.03(d,J=7.6Hz,1H).. 13 C NMR (125MHz, CDCl 3 ) : δ180.0, 179.1, 141.9, 138.8, 136.0, 135.3, 134.1, 130.8, 30.4, 130.1, 130.0, 128.9, 128.5, 128.4.
Embodiment 2
[0034]
[0035] Add 0.3mmol (E)-3-phenyl-1-(2-(phenylethynyl)phenyl)prop-2-en-1-one, 0.03mmol ferric nitrate, 0.6mmol diiodine pentoxide to 15mL In the thick-walled pressure-resistant reaction tube, add 3 mL of 1,4-dioxane as a solvent. Next, magnetic stirring was performed at 100° C. for 0.5 hours. After cooling to room temperature, add two tablespoons of column chromatography silica gel (100-200 mesh) to the reaction solution, and remove the solvent by distillation under reduced pressure, and then separate the product 3-phenyl-1,2-naphthoquinone by column chromatography Pure product (petroleum ether / ethyl acetate = 10:1 as eluent). The material was a red solid, 53% yield.
[0036] Characterization data: 1 H NMR (500MHz, CDCl 3 ):δ7.31–7.47(m,8H),7.58(m,1H,),8.03(d,J=7.6Hz,1H).. 13 C NMR (125MHz, CDCl 3 ) : δ180.0, 179.1, 141.9, 138.8, 136.0, 135.3, 134.1, 130.8, 30.4, 130.1, 130.0, 128.9, 128.5, 128.4.
Embodiment 3
[0038]
[0039] Add 0.3mmol (E)-3-phenyl-1-(2-(phenylethynyl)phenyl)prop-2-en-1-one, 0.015mmol ferric chloride, 0.6mmol diiodine pentoxide to In a 15mL thick-walled pressure-resistant reaction tube, add 3mL of 1,4-dioxane as a solvent. Next, magnetic stirring was performed at 100° C. for 0.5 hours. After cooling to room temperature, add two tablespoons of column chromatography silica gel (100-200 mesh) to the reaction solution, and remove the solvent by distillation under reduced pressure, and then separate the product 3-phenyl-1,2-naphthoquinone by column chromatography Pure product (petroleum ether / ethyl acetate = 10:1 as eluent). The material was a red solid, 43% yield.
[0040] Characterization data: 1 H NMR (500MHz, CDCl 3 ):δ7.31–7.47(m,8H),7.58(m,1H,),8.03(d,J=7.6Hz,1H).. 13 C NMR (125MHz, CDCl 3 ): δ180.0,179.1,141.9,138.8,136.0,135.3,134.1,130.8,30.4,130.1,130.0,128.9,128.5,128.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com