Matrine derivatives, synthesis thereof, and application thereof in control of plant diseases and pests
A derivative, matrine technology, applied in the field of agricultural protection, to achieve the effect of easy synthesis, good environmental compatibility and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of N-substituted-11-substituted matrine derivative compound 1 (method 1)
[0032]
[0033] method one
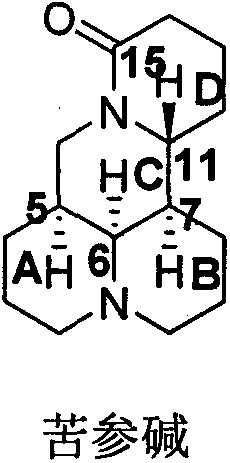
[0034] N-tert-butoxycarbonyl-11-dehydroxymatrine (C)
[0035] Extracted with ethyl acetate, combined the organic phases, washed with 80 mL of saturated brine, dried over anhydrous sodium sulfate, precipitated, and separated by column chromatography (DCM:MeOH=20:1) to obtain 3.00 g of a light yellow oily product with a yield of 81 %. Proceed directly to the next step.
[0036] 11-Dehydroxymatrine (D)
[0037] Extracted with ethyl acetate, combined the organic phases, washed with 80 mL of saturated brine, dried over anhydrous sodium sulfate, precipitated, and separated by column chromatography (DCM:MeOH=20:1) to obtain 1.20 g of a pale yellow oily product, which solidified in air. Yield 77%, Mp 46-47°C. 1 H NMR (400MHz, CDCl 3 )δ3.25(t, J=12.0Hz, 1H, NCH 2 ), 3.09-3.03 (m, 1H, NCH), 2.80 (dd, J = 16.0, 12.0Hz, 2H, NCH 2), 2.63 (dd, J=1...
Embodiment 2
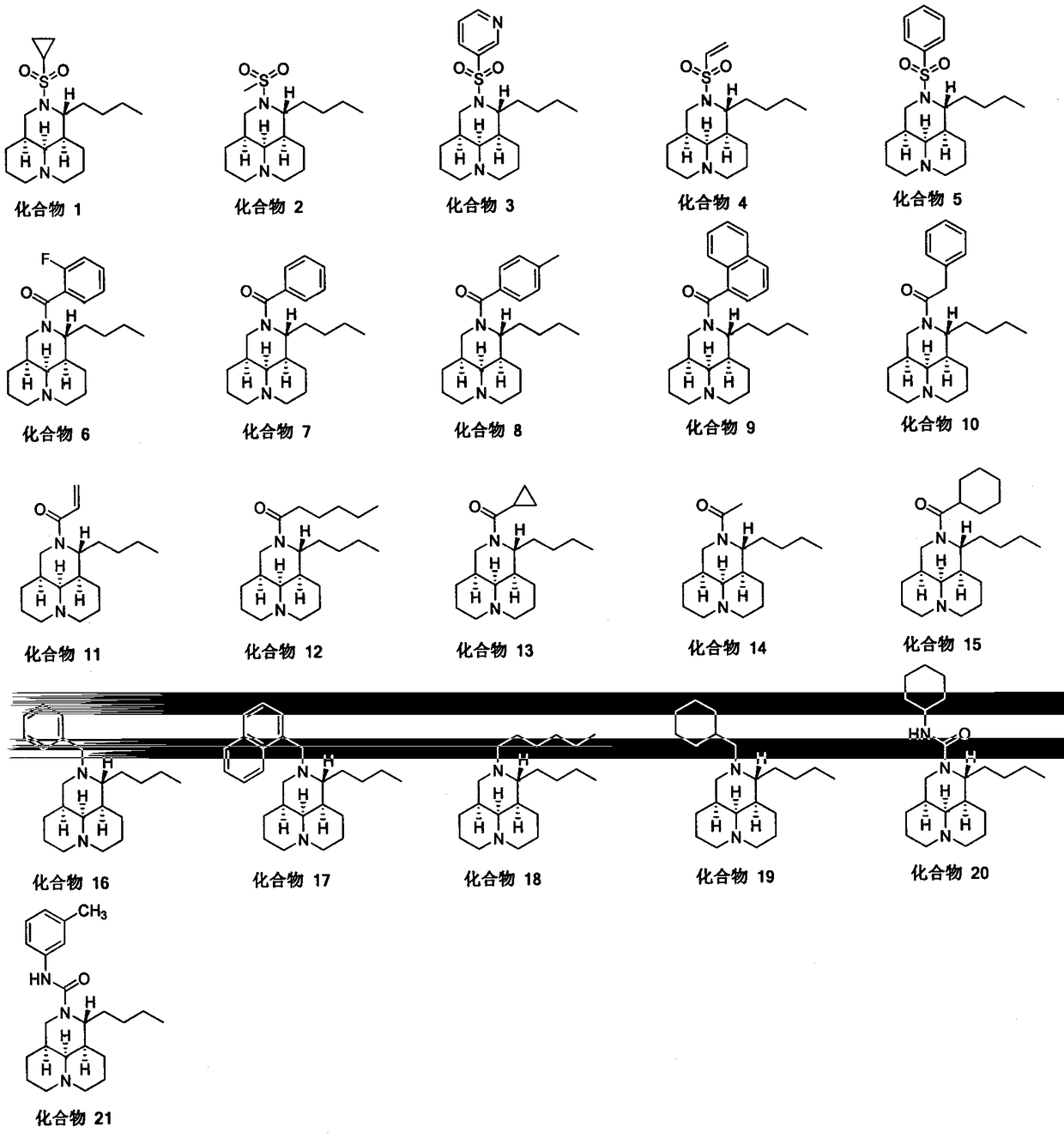
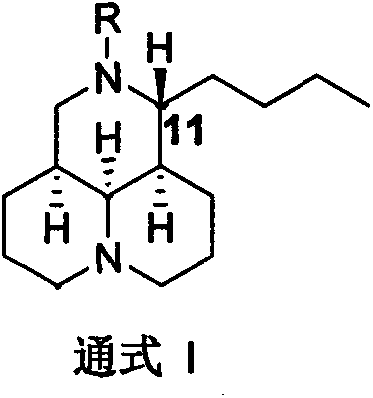
[0041] N-substituted-11 substituted matrine derivatives (compound 2-15) are completed with reference to the operation steps of Example 1 (see the attached figure 1 ):
[0042] Compound 2
[0043] White solid 0.30g, yield 75%, Mp 78-79°C. 1 H NMR (400MHz, CDCl 3 ) δ3.61 (d, J=4.0Hz, 1H, NCH), 3.44-3.39 (m, 1H, NCH 2 ), 3.29(t, J=12.0Hz, 1H, NCH 2 ), 2.91 (s, 3H, NCH 2 ), 2.74 (s, 2H, NCH 2 , NCH), 2.05-1.73 (m, 9H, CH 2 ), 1.59-1.26 (m, 10H, CH, CH 2 ), 0.92(t, J=4.0Hz, 3H, CH 3 ). 13 C NMR (100MHz, CDCl 3 )δ63.1, 57.3, 56.8, 56.7, 46.3, 39.7, 39.0, 34.2, 32.2, 28.4, 27.9, 27.7, 21.1, 20.9. HRMS (ESI) calcd for [C 16 h 30 N 2 o 2 S+H] + 315.2101, found 315.2190.
[0044] Compound 3
[0045] Yellow oily liquid, yield 76%. 1 H NMR (400MHz, CDCl 3 ) δ 9.07 (s, 1H, Py-H), 8.74 (d, J = 4.0Hz, 1H, Py-H), 8.14 (d, J = 8.0Hz, 1H, Py-H), 7.41 (dd, J=8.0, 4.0Hz, 1H, Py-H), 3.66 (dd, J=8.0, 4.0Hz, 1H, NCH), 3.53 (dd, J=8.0, 4.0Hz, 1H, NCH 2 ), 3.31-3.26 (m, 1H, NCH 2...
Embodiment 3
[0070] Example 3 Synthesis of N-substituted-11-substituted matrine derivatives (compound 16) (method 2)
[0071] N-substituted-11 substituted matrine derivatives (compounds 17-19) are completed by referring to the operation steps of Example 3 (see the accompanying drawings for the structural formula):
[0072]
[0073] Method Two
[0074] Compound 16
[0075] Add N-benzoyl-11-dehydroxymatrine (0.60g, 1.70mmol) to a 100mL round bottom flask, add lithium aluminum hydride (0.13g, 3.4mmol), heat up to reflux, and monitor the reaction by TLC after 6h Completely quenched in an ice-water bath, desolvated, added 20mL ethyl acetate, separated, the water phase was extracted with 3×40mL ethyl acetate, then washed with 60mL saturated brine, dried over sodium sulfate, desolventized to obtain a light yellow oily liquid 0.30 g, yield 79%. 1 H NMR (400MHz, CDCl 3 )δ7.34-7.20 (m, 5H, PhCH), 4.68 (s, 0.5H, NCH 2 Ph), 4.09 (d, J=12.0Hz, 0.5H, NCH 2 Ph), 3.04 (d, J=12.0Hz, 1H, NCH 2 Ph)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com