A kind of synthetic method of 2h-aziridine derivative
A synthetic method and allylidine technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of limited adaptability of reaction substrates, harsh conditions, complicated operation, etc., and achieve wide adaptability of substrates, mild reaction conditions, The effect of low process cost
Active Publication Date: 2021-08-24
NANCHANG HANGKONG UNIVERSITY
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Such a synthetic strategy has the disadvantages of multi-step reaction steps, complicated operation, harsh conditions, high cost, and limited adaptability of reaction substrates.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0031] The present invention will be further described in detail below in conjunction with specific embodiments.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
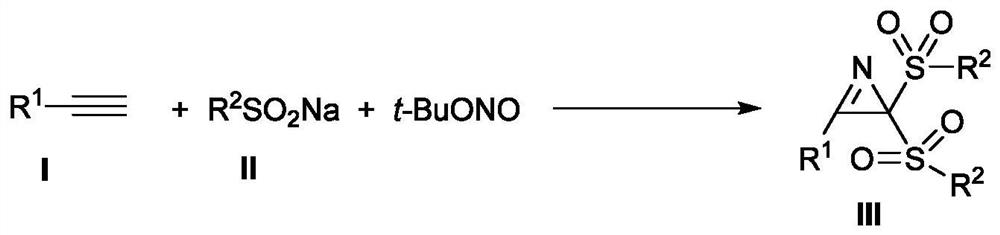
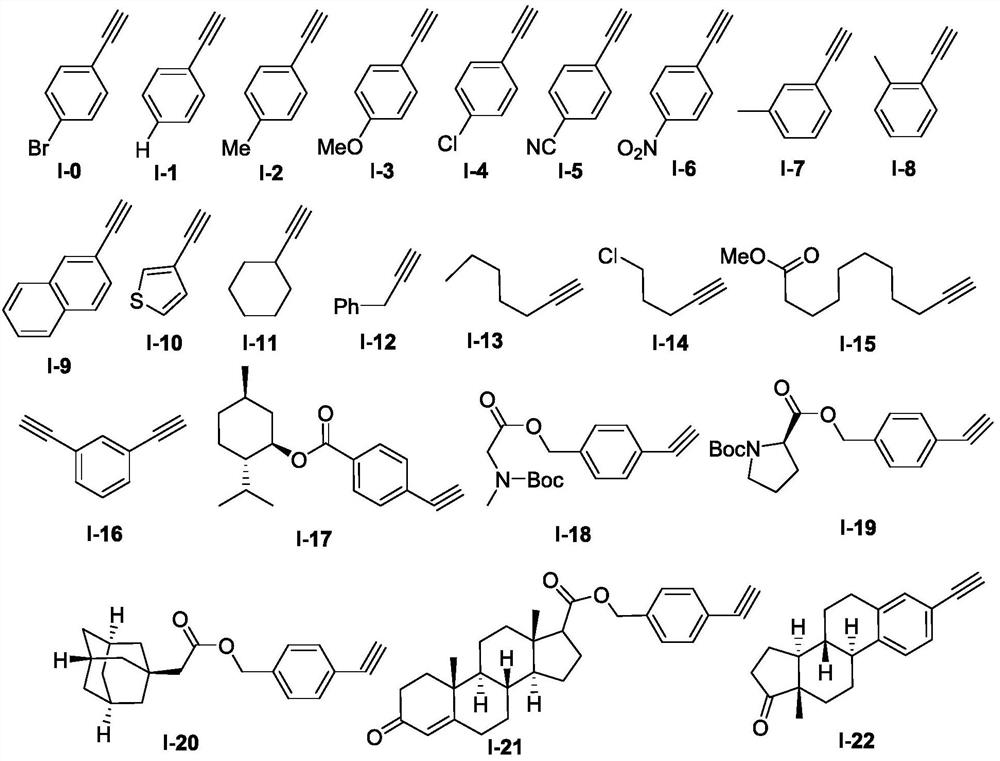
The invention discloses a new method for synthesizing 2H-aziridine derivatives. In the Schlenk sealed tube reactor, add the alkyne compound shown in formula I successively, the sulfonic acid sodium salt shown in formula II, add tert-butyl nitrite (t-BuONO) and organic solvent again, under inert atmosphere protection condition Next, the oil bath was heated and stirred to react, and after the completion of the reaction was monitored by TLC or GC-MS, the 2H-aziridine derivative shown in formula III was obtained through post-processing. The method has the advantages of easy source of raw materials, simple process route, mild reaction conditions, low process cost, wide substrate adaptability and high yield.
Description
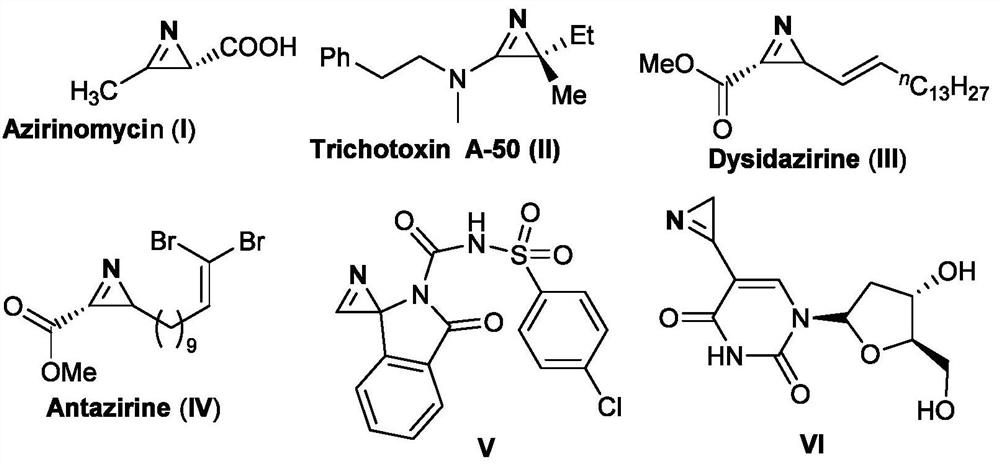
technical field [0001] The invention belongs to the technical field of organic synthesis, and in particular relates to a method for synthesizing 2H-azyridine derivatives. Background technique [0002] 2H-Aziridine is the smallest unsaturated azathree-membered ring structure existing in nature, and they widely exist in natural products, medicines, agricultural chemicals and organic materials, for example, the prior art has reported that ampicillin Azirinomycin and other compounds with the structures shown in the following formulas I-VI. [0003] [0004] Due to the widespread occurrence of 2H-aziridine derivatives in nature and their great potential in the construction of amino derivatives and nitrogen-containing heterocycles, chemists have conducted intensive research on this class of molecules. 2H-Aziridine derivatives have been widely used in the synthesis of complex nitrogen-containing acyclic molecules and various nitrogen heterocyclic compounds, such as pyrroles, in...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D203/04C07D409/04C07J43/00
CPCC07D203/04C07D403/04C07D409/04C07J43/003
Inventor 胡明何兴一吴双李金恒
Owner NANCHANG HANGKONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com