Alkaline aluminum-air battery electrolyte with fluorescent effect, and addition thereof
An electrolyte additive, empty battery technology, applied in the field of electrochemistry, can solve the problems of poor stability of aluminum ions, weak recognition of aluminum ions, unfavorable complexes, etc. Forming, easy to fall off effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The chemical formula of 8-hydroxyquinoline in the examples is 8-HQ, where mM represents millimoles and M represents mol.
[0052] In the aluminum-air battery electrolyte compound additive in this embodiment, the electrolyte is a 4 mol / L sodium hydroxide solution, and the additive is 8-HQ with a concentration of 2.5-10 mM / L. The preparation method of the electrolyte is: preparing a sodium hydroxide solution with a concentration of 4M / L, cooling to room temperature, adding 8-HQ, and ultrasonic stirring for dissolution.
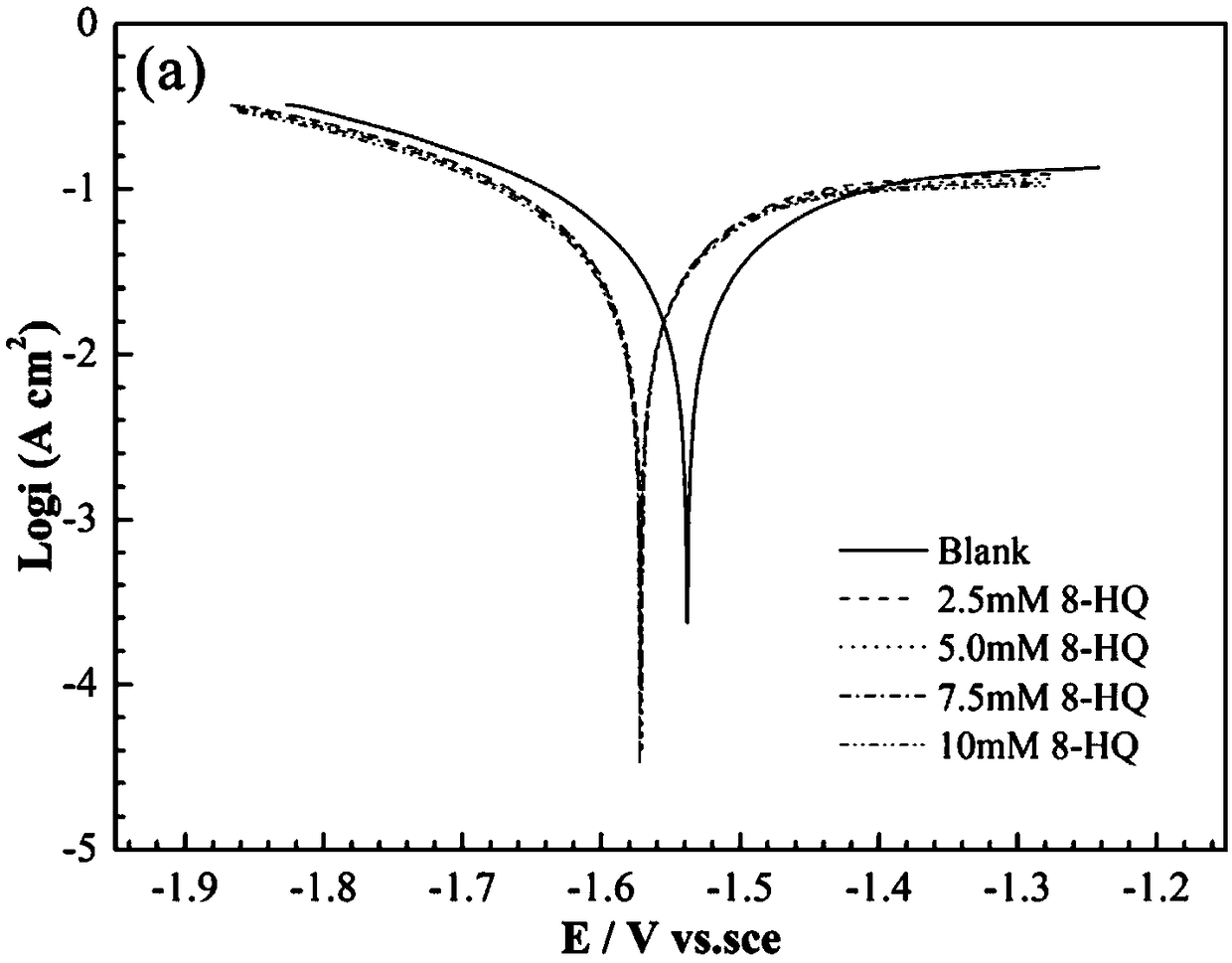
[0053] The gas collection method was used to test the hydrogen evolution self-corrosion rate of AA5052 aluminum alloy in the electrolyte prepared in this embodiment. The test time was 30 minutes. The results are shown in Table 1. The performance of A5052 aluminum alloy anode in 4mol / L sodium hydroxide solution containing different additives The polarization curve fitting data are shown in Table 2.
[0054] Table 1 Hydrogen evolution corrosion of AA5052 aluminum...
Embodiment 2
[0064] In the aluminum-air battery electrolyte compound additive in this embodiment, the electrolyte is a 4 mol / L sodium hydroxide solution, and the additive is ZnO with a concentration of 1.0-8.0 mmol / L. The preparation method of the electrolyte is: preparing a sodium hydroxide solution with a concentration of 4 mol / L, cooling to room temperature, adding ZnO, and stirring to dissolve.
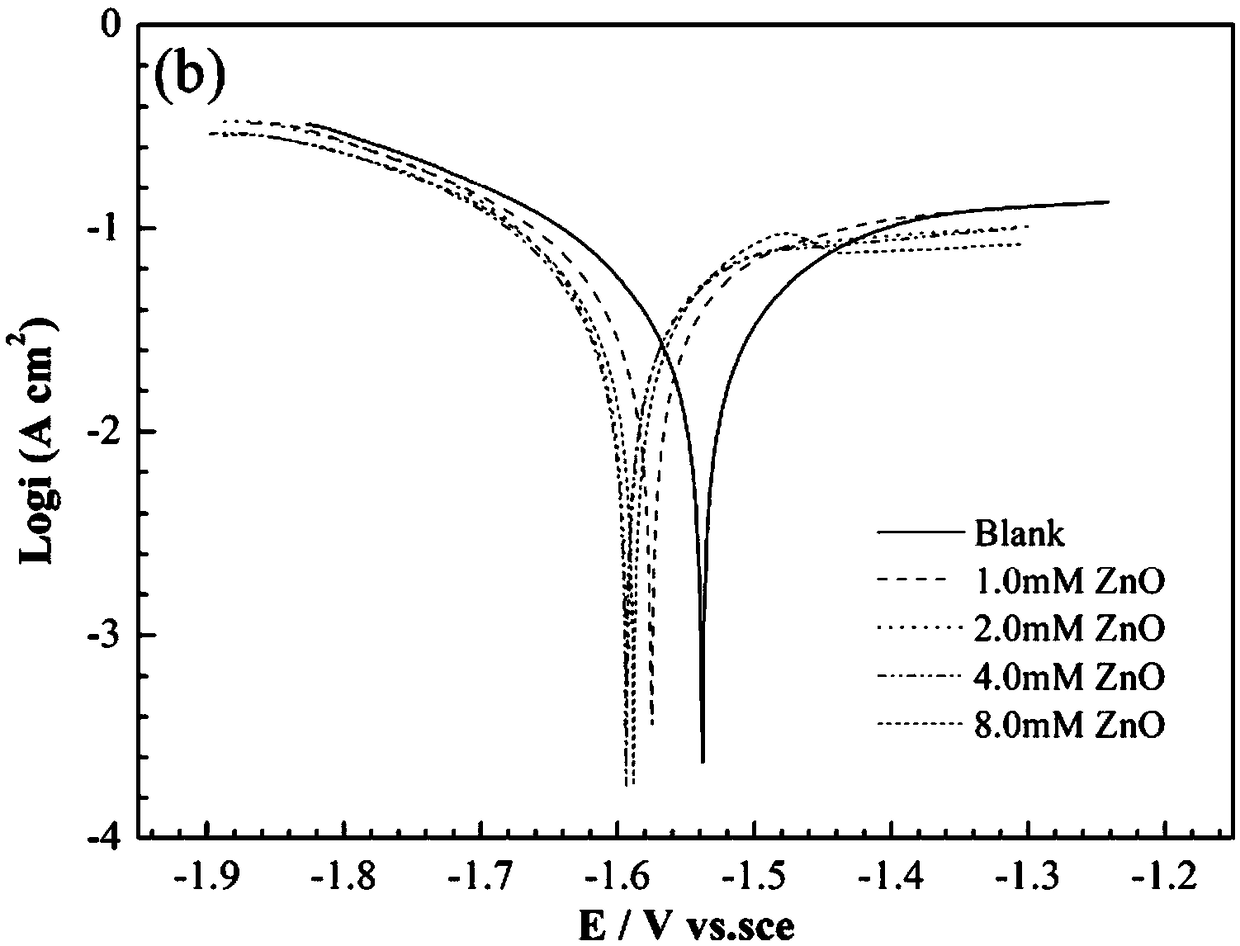
[0065] The gas collection method was used to test the hydrogen evolution self-corrosion rate of the AA5052 aluminum alloy in the electrolyte prepared in this embodiment, and the test time was 30 minutes. The results are shown in Table 1. The polarization curve and AC impedance of AA5052 aluminum alloy in the above electrolyte are tested by electrochemical test, and the results are shown in Figure 1b with Figure 2b , The temperature is controlled at 25°C.
[0066] From Table 1, Table 2, Figure 1b with Figure 2b It can be seen that after adding ZnO, the corrosion potential shifts negatively, the ...
Embodiment 3
[0068] The aluminum-air battery electrolyte compound additive in this embodiment, wherein the electrolyte is a 4mol / L sodium hydroxide solution, the additive adopts 8-HQ with a solubility of 2.5-10 mmol / L, and the compound has a solubility of ZnO It is 4.0mmol / L. The preparation method of the electrolyte is as follows: prepare a sodium hydroxide solution with a concentration of 4mol / L, cool to room temperature, add 8-HQ with different concentrations, and after ultrasonically dissolve completely, add ZnO to make the concentration 4.0mmol / L, stir to dissolve .
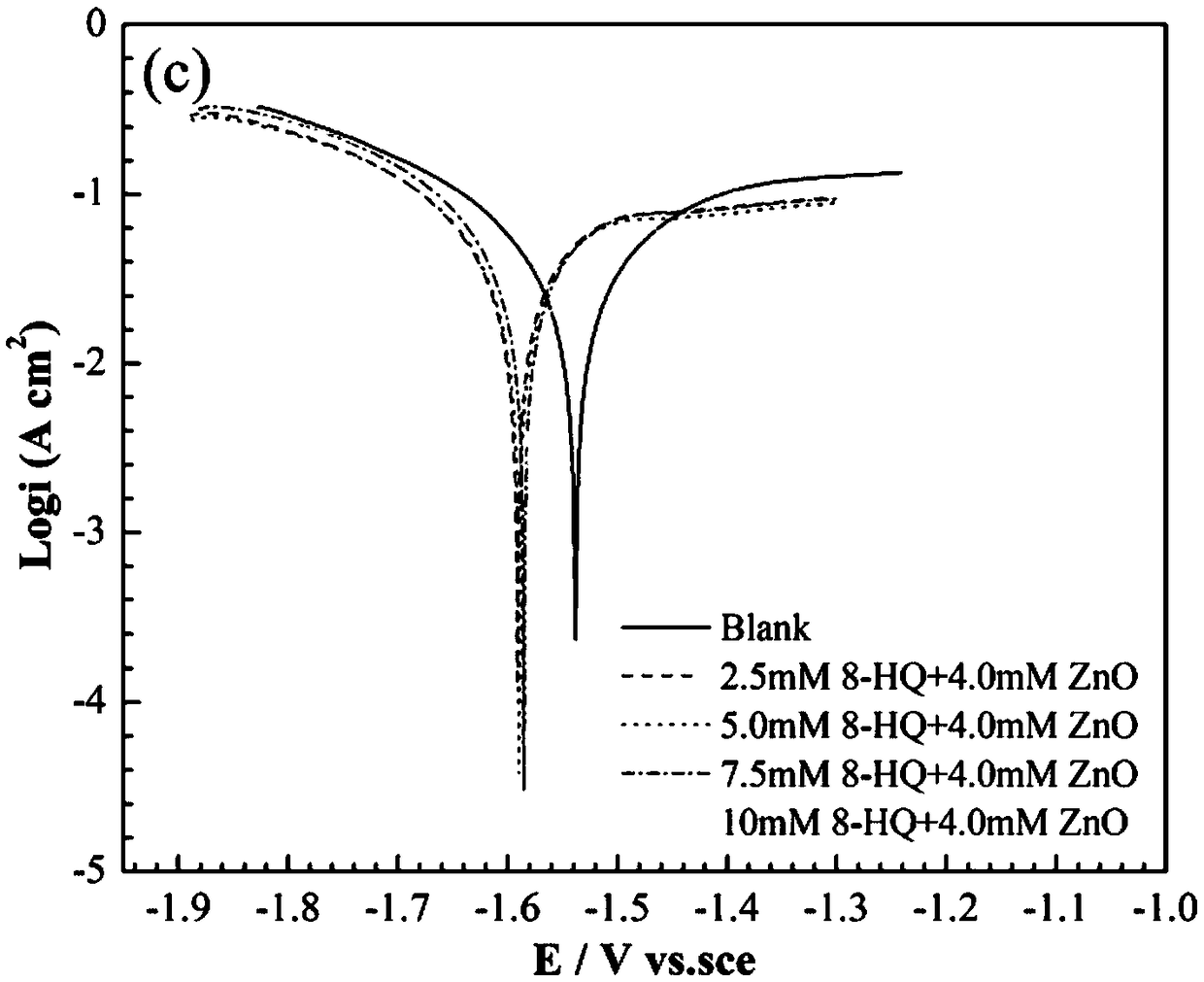
[0069] The gas collection method was used to test the hydrogen evolution self-corrosion rate of the AA5052 aluminum alloy in the electrolyte prepared in this embodiment, and the test time was 30 minutes. The results are shown in Table 1. The polarization curve and AC impedance of AA5052 aluminum alloy in the above electrolyte are tested by electrochemical test, and the results are shown in Figure 1c with Figure 2c , The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com