A kind of cetirizine hydrochloride tablet and preparation method thereof
A technology of cetirizine hydrochloride and rizine tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the risk of explosion, stickiness, complicated preparation process, etc. Problems, to achieve the effect of small decrease in dissolution rate, not easy to compress, and rapid dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
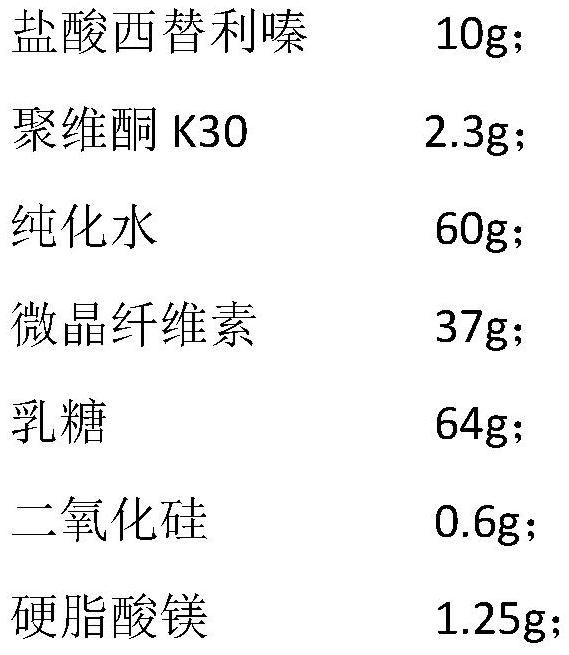
[0042] Embodiment 1 provides a kind of cetirizine hydrochloride tablet, and its preparation raw material comprises following component:
[0043]
[0044] The average particle size of the microcrystalline cellulose is 80 mesh (about 180 microns).
[0045] Preparation process: Dissolve cetirizine hydrochloride in povidone K30 aqueous solution, put microcrystalline cellulose into fluidized bed, use cetirizine hydrochloride aqueous solution to perform fluidized bed granulation, dry and granulate to obtain cetirizine hydrochloride Lizine pretreatment granules are then uniformly mixed with prescription amounts of lactose, silicon dioxide and magnesium stearate, and compressed into tablets.
Embodiment 2
[0047] Embodiment 2 provides a kind of cetirizine hydrochloride tablet, and its preparation raw material comprises following component:
[0048]
[0049] The average particle size of the microcrystalline cellulose is 80 mesh (about 180 microns).
[0050] Preparation process: Dissolve cetirizine hydrochloride in povidone k30 water, put microcrystalline cellulose into a high-efficiency wet granulator, use cetirizine hydrochloride aqueous solution for wet granulation, dry and granulate to obtain cetirizine hydrochloride The granules are pretreated, then mixed evenly with the prescribed amount of croscarmellose sodium, mannitol, silicon dioxide and magnesium stearate, and compressed into tablets.
Embodiment 3
[0052] Embodiment 3 provides a kind of cetirizine hydrochloride tablet, and its preparation raw material comprises following component:
[0053]
[0054] The average particle size of the microcrystalline cellulose is 100 mesh (about 150 microns).
[0055] Preparation process: Dissolve cetirizine hydrochloride in povidone k30 water, put microcrystalline cellulose into a high-efficiency wet granulator, use cetirizine hydrochloride aqueous solution for wet granulation, dry and granulate to obtain cetirizine hydrochloride The granules are pretreated, then mixed evenly with the prescribed amount of croscarmellose sodium, mannitol, silicon dioxide and magnesium stearate, and compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com