Chalcone derivatives containing allyl structures, and application of chalcone derivatives
A technology of chalcone derivatives and allyl groups, which is applied in the direction of medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve problems such as unavoidable side effects, and achieve a good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~39
[0027] The synthesis of embodiment 1~39 compound:
[0028] The method for the chalcone derivatives containing allyl structure prepared by the present invention, the synthetic general route is as follows formula a:
[0029]
[0030]
[0031] Synthetic steps (steps a-e) of compounds (1,3-8,10-30) in type I:
[0032] Step a Synthesis of 2a: Potassium carbonate (68.1 g, 0.48 mol) was slowly added to 4-hydroxy-2-methoxybenzaldehyde (1a; 25.0 g, 0.16 mol) and 3-bromoprop-1-ene (38.1 g , 0.32mol) in acetone solution, the reaction mixture was refluxed for 6 hours. The resulting mixture was filtered, and the filtrate was concentrated under reduced pressure. The residue was then dissolved in ethyl acetate (50 mL), washed with water (50 mL) and brine (50 mL). MgSO for organic layer 4 Dry, filter, and concentrate under reduced pressure. The residue was purified by silica gel chromatography to obtain compound 2a (27.95 g, 91%) as a pale yellow liquid.
[0033] Step b Synthesis ...
Embodiment 29
[0078] The chemical structure characterization data of compound 2 synthesized in Example 29
[0079] Compound 2: (E)-3-{5-allyl-2-methoxy-4-[(tetrahydro-2H-pyran-2-yl)oxy]phenyl}-1-(4- (E)-3-{5-Allyl-2-methoxy-4-[(tetrahydro-2H-pyran-2-yl)oxy]phenyl}-1- (4-hyd roxyphenyl)prop-2-en-1-on. Yellow liquid, yield 45.3%, 1 H NMR (500MHz, acetone--d 6 )δ (ppm): 8.98 (s, 1H), 8.71 (s, 1H), 7.90 (d, J = 15.7Hz, 1H), 7.88 (d, J = 8.6Hz, 2H), 7.50 (d, J = 15.6Hz, 1H), 7.47(s, 1H), 6.8(d, J=8.5Hz, 1H), 6.47(s, 1H), 5.90(ddt, J=16.7, 10.1, 6.5Hz, 1H), 5.76( m,1H),4.93(dd, J=17.1,1.7Hz,1H),4.87(d,J=8.9Hz,1H),3.74(s,3H),3.60(t,J=8.6Hz, 2H), 3.22(d, J=6.5Hz, 2H), 2.22(m, 2H), 1.93(m, 2H), 1.71(m, 2H). 13 C-NMR (125MHz, acetone-d 6 )δ (ppm): 188.4, 162.3, 159.7, 159.2, 139.2, 138.1, 131.7, 131.5×2, 131.4, 120.1, 119.5, 116.6, 116.0×2, 102.3, 115.4, 99.9, 62.4, 56.0, 34.2, 24.6. ESI-MS m / z:395.18(M+H) + , calcd for C 24 h 26 o 5 :394.18.
[0080] Compound 9: (E)-3-{5-allyl-2-methoxy-...
Embodiment 40
[0098] Example 40 Activity Test
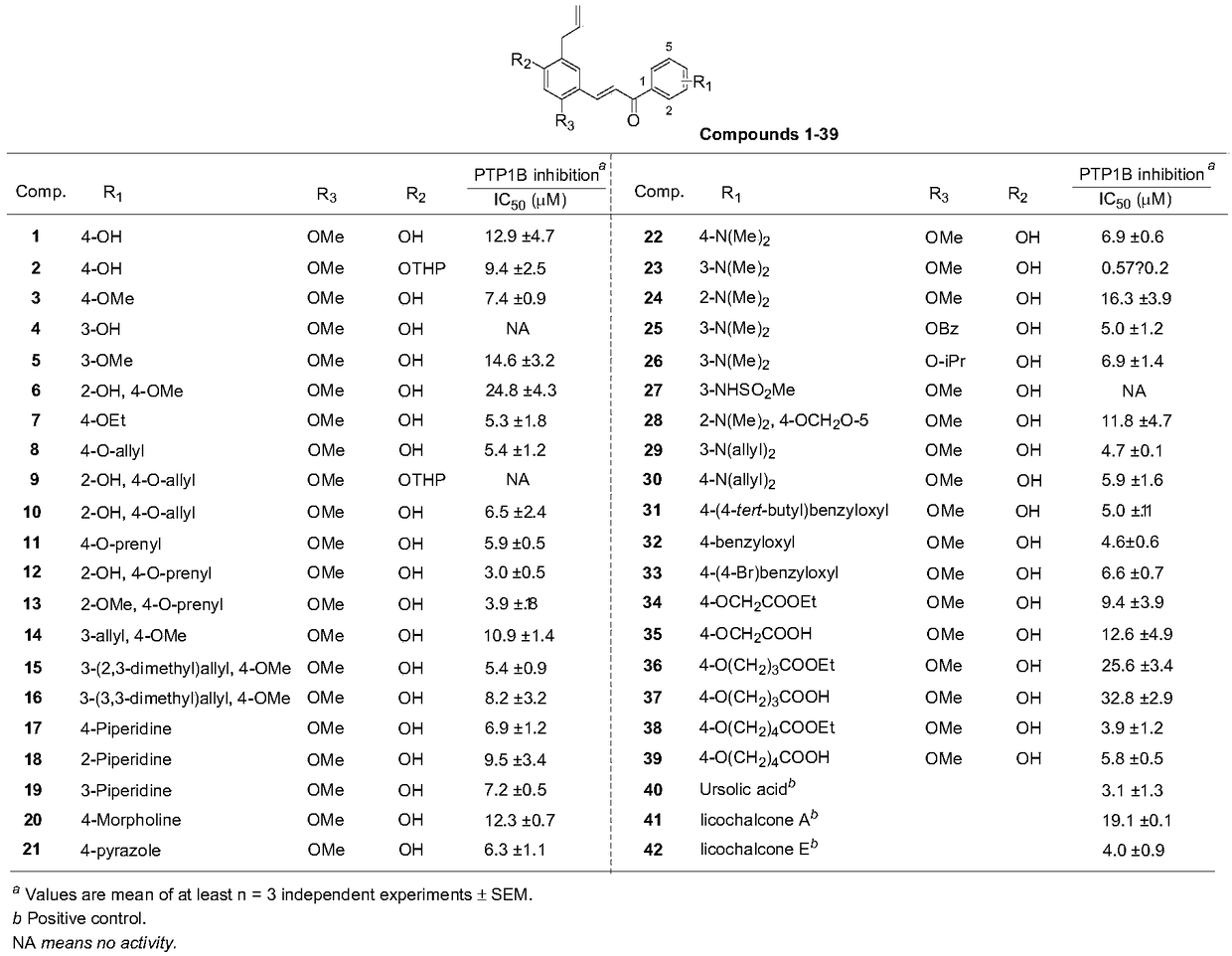
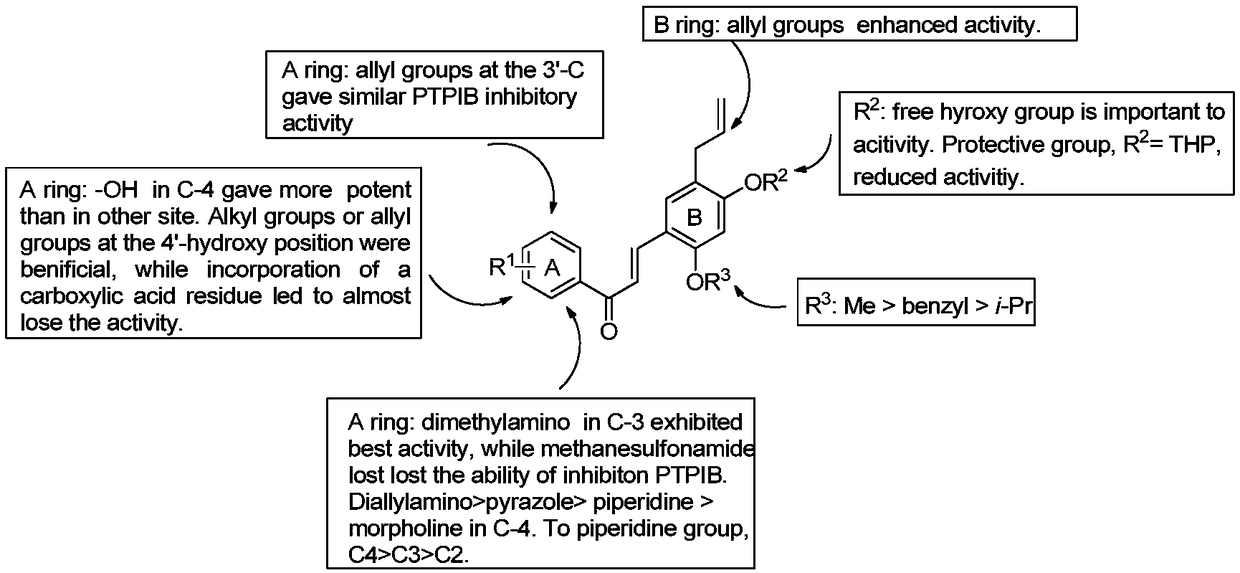
[0099] With p-nitrophenyl phosphate (pNPP) as a substrate, the inhibitory activity of the compounds prepared in Examples 1 to 39 of the present invention on PTP1B was determined, and the results are summarized in figure 1middle. A known PTP1B inhibitor, ursolic acid (IC50 = 3.1 M), was used as a positive control. Except compounds 4, 9, 27 and 37, all synthetic compounds dose-dependently inhibited PTP1B activity with IC50 values ranging from 0.5 to 24.8 μM. Most of these compounds showed better activity than licochalcone. Among them, compound 1 showed twice the inhibitory activity compared with licochalcone A. Compound 7 (IC50=5.3×M), 8 (IC50=5.4×M) and 11 (IC50=5.8×M), compared with compound 1, the inhibitory activity increased about 2 times. As the length of 4-hydroxyl substitution increases, it shows proportionally increased inhibitory potency against PTP1B (especially compound 12 with isoprenyl group), exhibiting particularly good act...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com