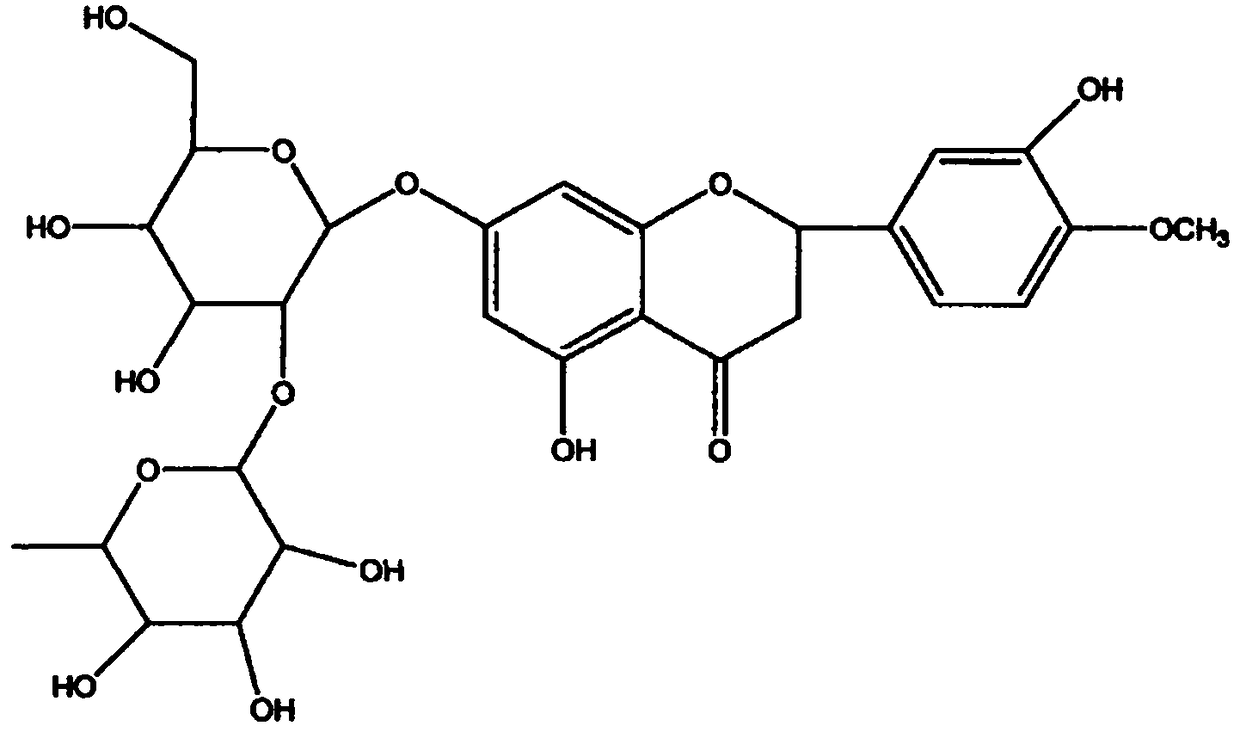
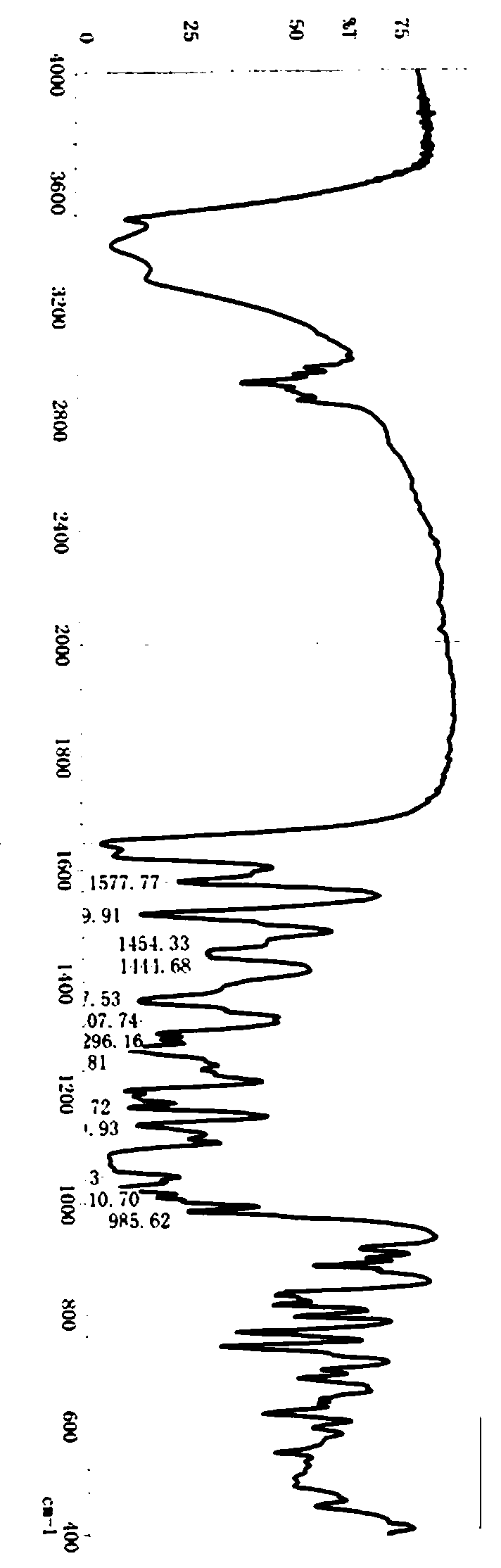
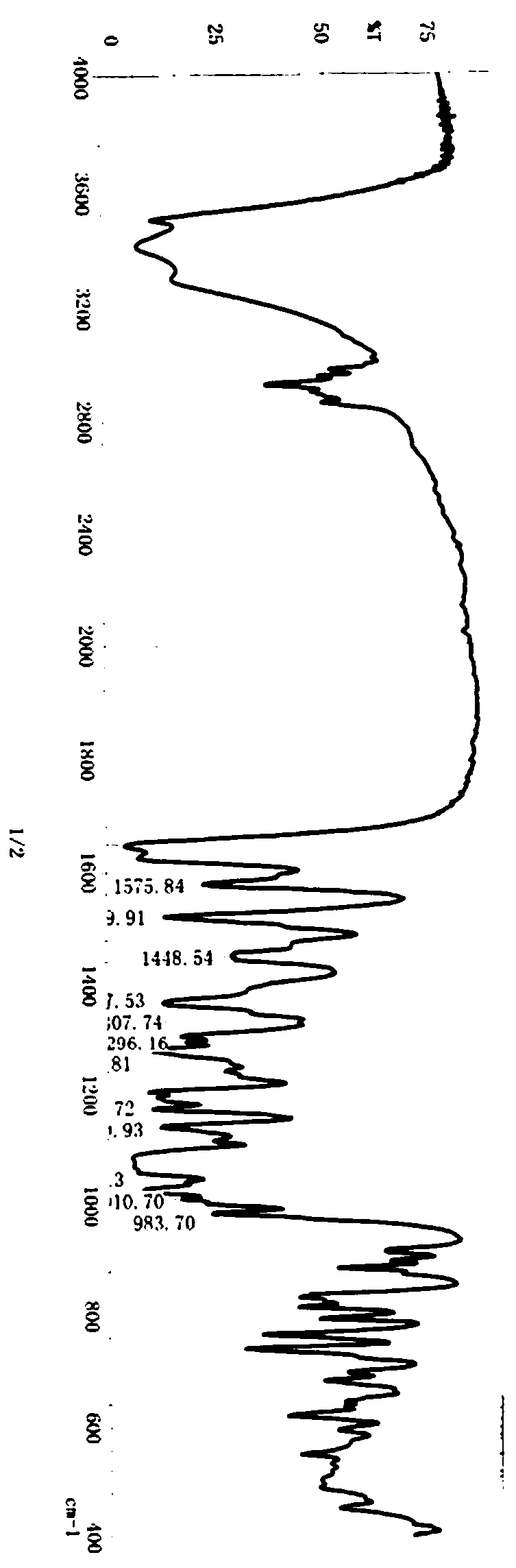
Method for synthesizing neohesperidin by using citrus paradisi macfadyen
A technology of pomelo fruit extract and neohesperidin, which is applied in the field of synthesizing neohesperidin from pomelo fruit extract, can solve the problems of long production cycle, high cost, and complicated operation, so as to improve quality and yield, and improve product quality. The effect of high quality, yield and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for synthesizing neohesperidin with pomelo fruit extract, comprising the steps of:
[0033] 1) Hydrolysis:
[0034] ① Add pure water to the crude pomelo fruit extract under stirring conditions, then add dilute alkali solution, control the final alkali concentration to 10%, and keep it warm at 100°C for 5 hours;
[0035] ②After the reaction, add a small amount of ice water to lower the temperature of the reaction solution to 40°C, adjust the pH, and crystallize at a temperature of 0-20°C for 24 hours;
[0036] ③ Filter, wash with water until the eluate is clear, and drain;
[0037] ④Appropriate amount of hot water is dissolved, and the activated carbon is decolorized;
[0038] ⑤ Filtrate, refrigerate and crystallize overnight, filter, wash with water until the eluate is clear, and drain to obtain root bark acetophenone-4'-β-neohesperidoside;
[0039] 2) Synthesis:
[0040] ①After dissolving root bark acetophenone-4'-β-neohesperidoside with 5 times the weight...
Embodiment 2
[0047] A method for synthesizing neohesperidin with pomelo fruit extract, comprising the steps of:
[0048]Add pure water to the crude pomelo fruit extract under stirring conditions, then add dilute alkali solution, control the final alkali concentration to 20%, and keep it warm at 120°C for 10 hours; after the reaction, add 3 times the amount of ice water to reduce the temperature of the reaction solution Reduce to 40°C, use hydrochloric acid to adjust the pH to 2~7, crystallize at a temperature of 0~20°C for 24 hours; filter, wash with water until the eluate is clear, and drain; dissolve in appropriate amount of hot water, decolorize with activated carbon; filter, refrigerate and crystallize Overnight, filter, wash with water until the eluate is clear, and drain to obtain root bark acetophenone-4'-β-neohesperidoside; use 5 times the weight of organic solvent to dissolve root bark acetophenone-4'-β-neohesperidoside After glucoside, add 0.1 times of glacial acetic acid and 0.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com