Guided bone regeneration device
A guided bone regeneration and biological technology, applied in medical science, dentistry, dental repair, etc., can solve the problems of maintaining bone repair space, additional trauma, iatrogenic complications, etc., to avoid additional trauma and iatrogenic complications Symptoms, good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
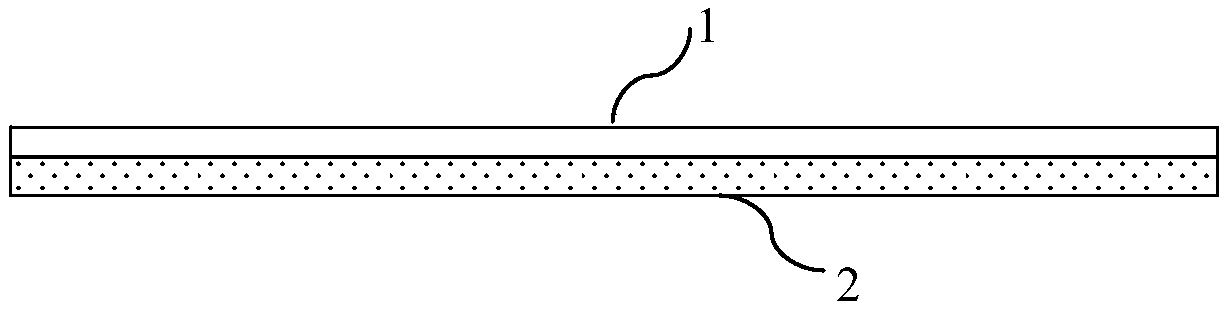
[0026] This embodiment provides an exemplary guided bone regeneration device with a double-layer structure. like figure 1 As shown, the guided bone regeneration device of this embodiment is composed of a layer of support layer 2 and a guide bone regeneration membrane 1 arranged on the entire surface of one side of the support layer 2, wherein the guide bone regeneration membrane 1 is used to cover the Repair parts. Wherein, the guiding bone regeneration membrane 1 and the support layer 2 are connected by bonding, and the guiding bone regeneration membrane 1 is made of fibrin into a planar sheet by 3D printing. The supporting layer 2 is made of polycaprolactone (PCL) by electrospraying into a planar sheet with a thickness of 1 mm.
[0027] The guided bone regeneration device of this example has a certain degree of support. At the same time, the fibrin and PCL materials used to make the guided bone regeneration membrane and support layer of the guided bone regeneration device...
Embodiment 2
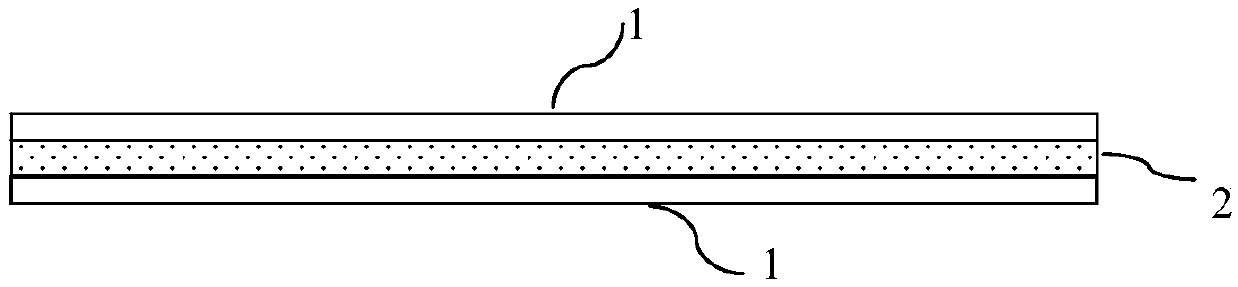
[0029] This embodiment provides an exemplary guided bone regeneration device with a sandwich structure. like figure 2 As shown, the guided bone regeneration device of the present embodiment consists of one deck of support layer 2 and one deck of guide bone regeneration film 1 respectively arranged on the entire surface of the support layer 2 both sides (that is, guide bone regeneration film-support layer - structure of the membrane that guides bone regeneration). Wherein, the guided bone regeneration membrane 1 is formed by electrospinning of collagen and made into a planar sheet. The support layer 2 is made of polyglycolic acid (PGA) into a grid shape by electrospinning, and its thickness is 1.5 mm. The supporting layer 2 and the guiding bone regeneration membrane 1 arranged on both sides thereof are connected by suturing with absorbable sutures, thereby forming a sandwich structure guiding bone regeneration device.
[0030] The guided bone regeneration device of this exa...
Embodiment 3
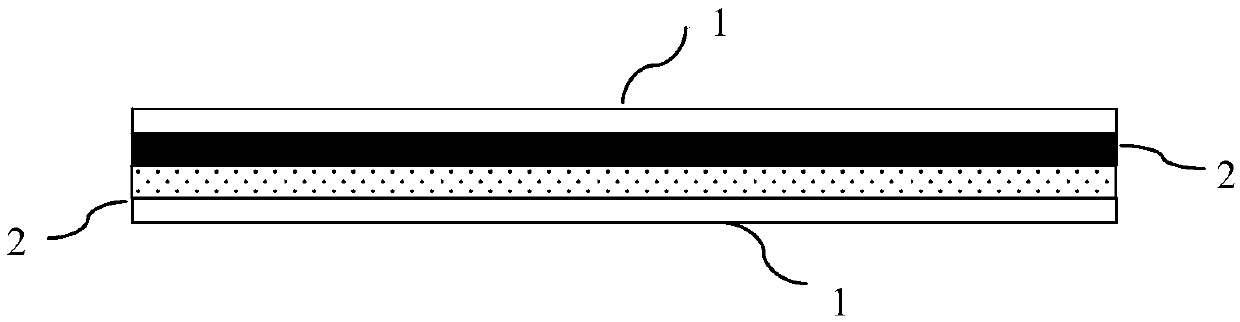
[0032] This embodiment provides an exemplary guided bone regeneration device with a four-layer structure. like image 3 As shown, the guided bone regeneration device of this embodiment is composed of two laminated support layers 2 and guided bone regeneration membranes 1 respectively arranged on the entire surfaces of both sides of the laminated support layers, that is, guided bone regeneration membrane-support layer - Support layer - the structure of the membrane that guides bone regeneration. Among them, the guided bone regeneration membrane 1 is made of polysaccharide and formed into a planar sheet by 3D printing. The supporting layer 2 is made of polylactic-glycolic acid (PLGA), and formed into a strip shape by electrospinning, and its thickness is 0.5 mm. In addition, in this embodiment, the bone-guiding regeneration film further includes calcium phosphate with a weight percentage of 40%, and the supporting layer further includes calcium phosphate with a weight percentage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com