Process for preparing Hongjin stasis elimination tablet
A preparation process and a technology for eliminating junction tablets, which are applied in the field of pharmacy and can solve the problems of insufficiently clear spot color, long production cycle, and unfavorable large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Hongjin Xiaojie Tablets include raw materials: The Hongjin Xiaojie Tablets include raw materials: Spiced Spathiphyllum, Jiyateng, Golden Buckwheat, Dahongpao, Bupleurum, Panax notoginseng, Cyperus cyperi, Star anise lotus, Ratwort and Black ants; Among them, Panax notoginseng was purchased from Nanguo Trading Co., Ltd., Wenshan City, Yunnan Province, batch number: 20170901; Bupleurum was purchased from Rongxian Minzhong Traditional Chinese Medicine Planting Professional Cooperative, batch number is 20170801; Lot No.: 20170702), Ratwort (Lot No.: 20170703), Black Ant (Lot No.: 20170704), Spiced Bloodvine (Lot No.: 20170705), Chicken Yateng (Lot No.: 20170706), Golden Buckwheat (Lot No.: 20170707) and Dahongpao ( Batch number: 20170708) were purchased from Guang'an Xinsi Planting Professional Cooperative.
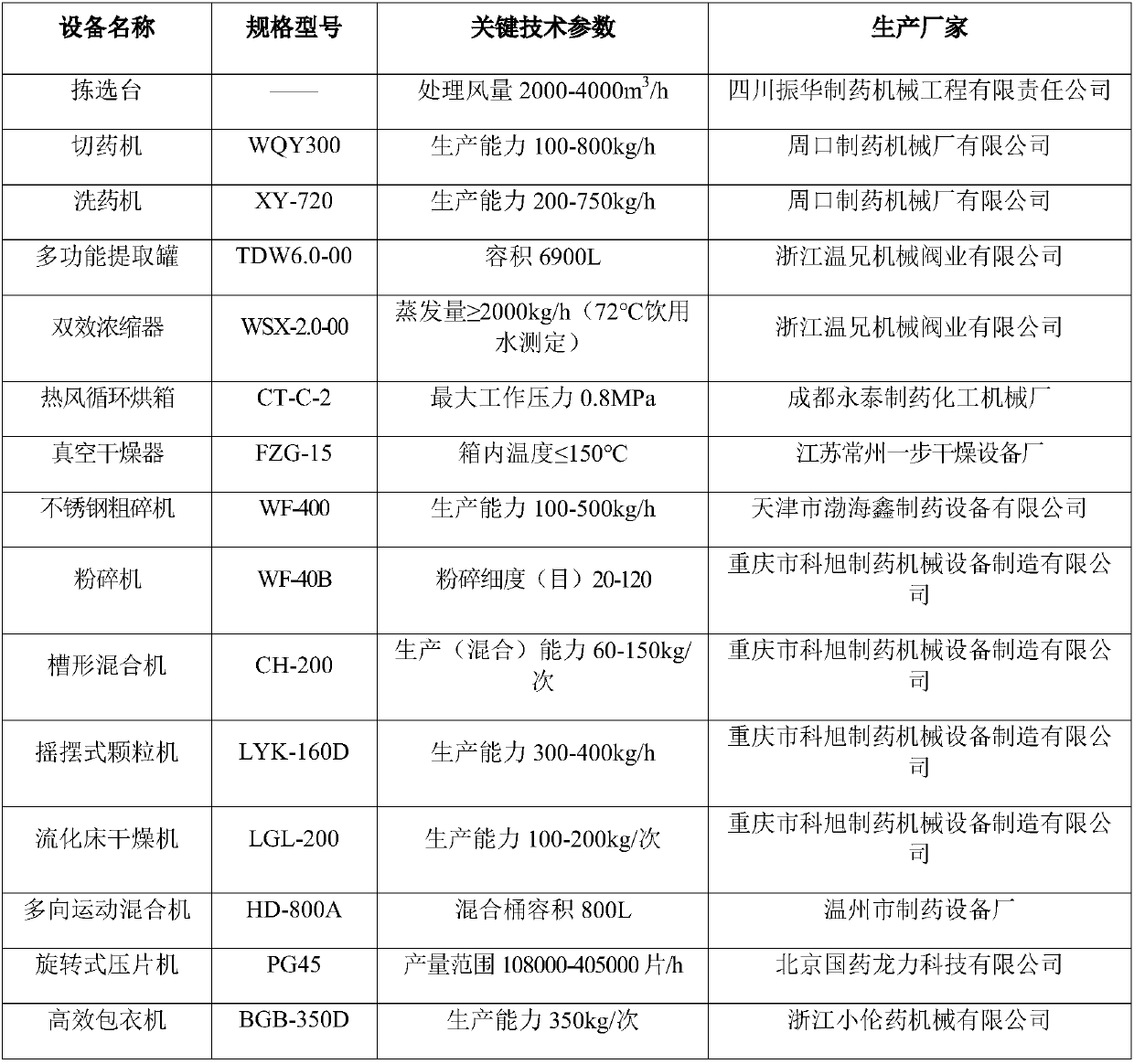
[0059] Main instruments and equipment of the present invention are as table 2:
[0060] Table 2 main equipment
[0061]
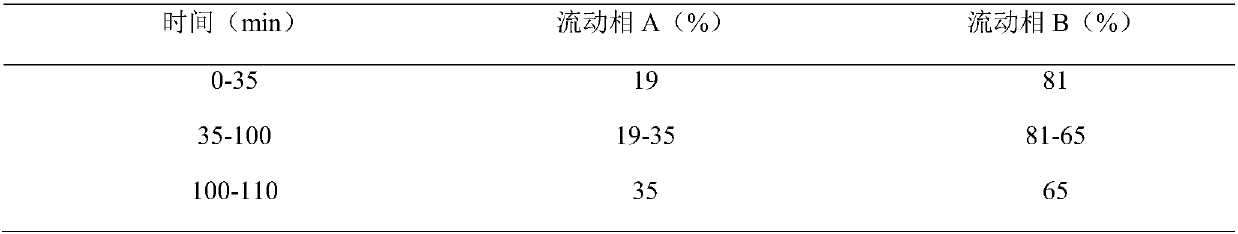
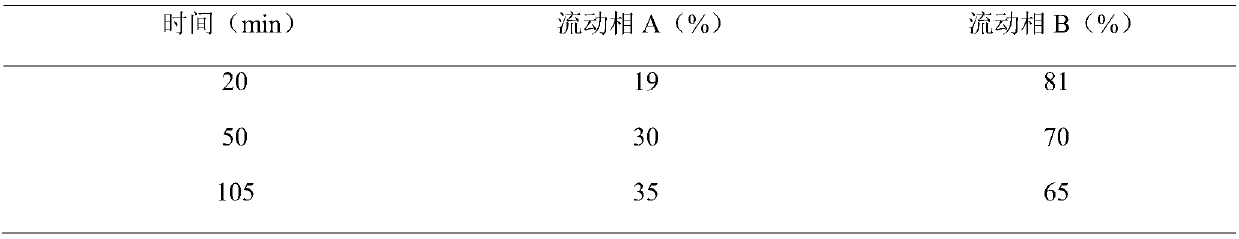
[0062] The preparation method of every 1...
Embodiment 2
[0085] The difference with Example 1 is that the weight of the dry paste powder is adjusted to be 232.5g, and the weight of dextrin is 0g
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com