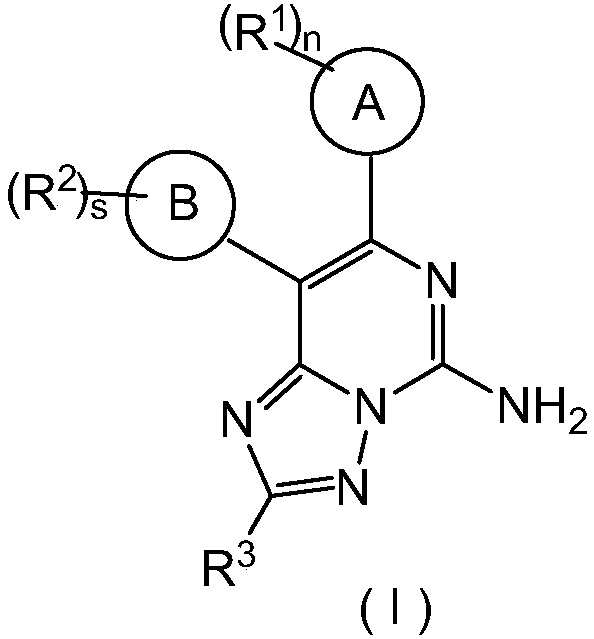
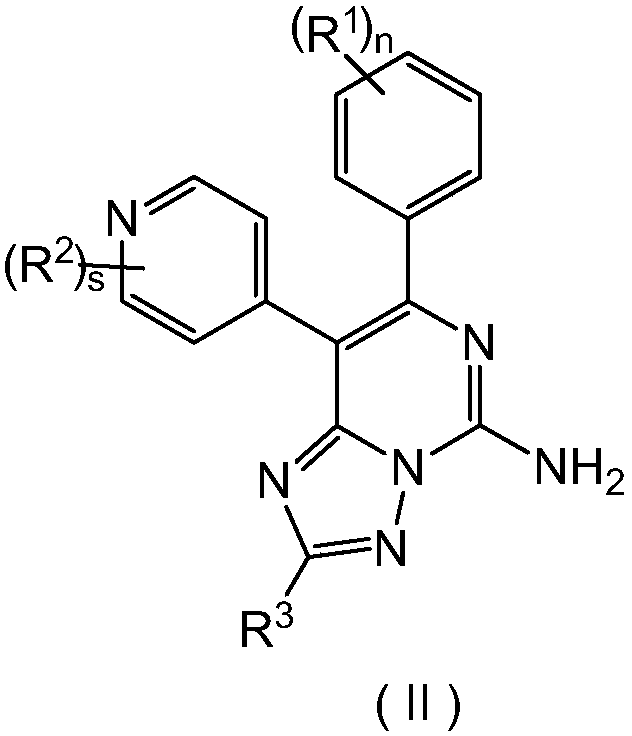
Triazolopyrimidine derivative, preparation method thereof, and application thereof in medicines
A technology of compounds and general formulas, which can be used in pharmaceutical formulations, antipyretics, drug combinations, etc., and can solve the problems of low activity, low selectivity, and low bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
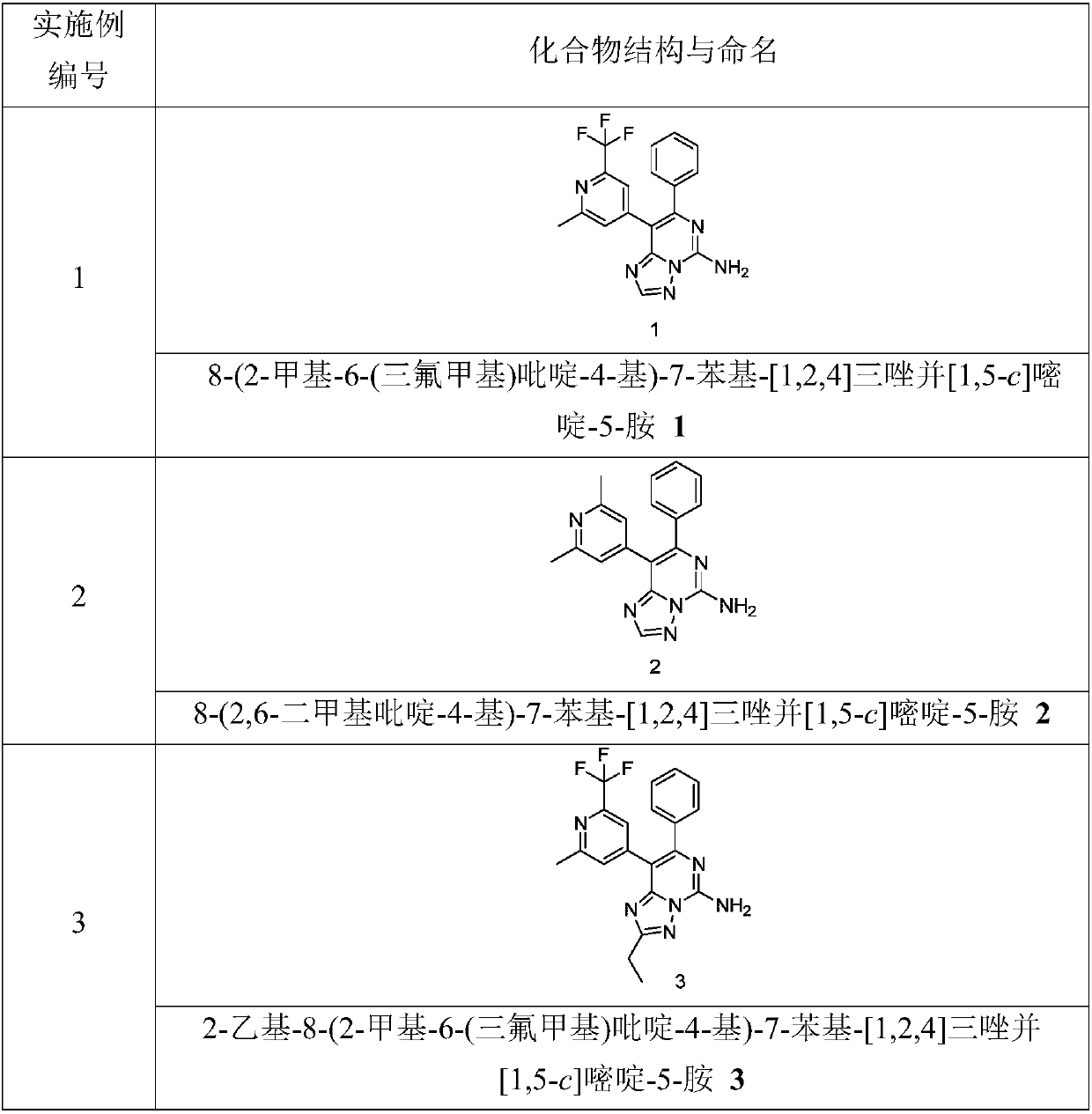
[0236] 8-(2-Methyl-6-(trifluoromethyl)pyridin-4-yl)-7-phenyl-[1,2,4]triazolo[1,5-c]pyrimidin-5-amine 1
[0237]
[0238]
[0239] first step
[0240] 2-Methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-6-(trifluoromethyl)pyridine 1b
[0241] Under a nitrogen atmosphere, (1,5-cyclooctadiene)methoxyiridium(I) dimer (1.0 g, 1.55 mmol), 4,4,4',4',5,5,5 ',5'-octamethyl-2,2'-bis(1,3,2-dioxaborolane) (15.7 g, 62.1 mmol) and 4,4'-di-tert-butyl-2, 2'-Dipyridine (0.83g, 3.1mmol) was dissolved in 100mL of n-hexane, heated to 50°C, stirred for 10 minutes, and 2-methyl-6-(trifluoromethyl)pyridine 1a (5g, 31mmol, Prepared by the known method "Chemical and Pharmaceutical Bulletin, 1990, 38(9), 2446-2458"), and continued stirring for 4 hours. The reaction was stopped, cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by thin-layer chromatography with developer system B to obtain the title compound 1b (6 g, y...
Embodiment 2
[0267] 8-(2,6-Dimethylpyridin-4-yl)-7-phenyl-[1,2,4]triazolo[1,5-c]pyrimidin-5-amine 2
[0268]
[0269] first step
[0270] 5-Bromo-4-chloro-6-phenylpyrimidin-2-amine 2a
[0271] Compound 1e (13g, 7.34mmol) was dissolved in 300mL N,N-dimethylformamide, N-bromosuccinimide (12.38g, 69.54mmol) was added, and the reaction was stirred for 1 hour. The reaction was stopped, the reaction solution was poured into 1L of water, stirred for 20 minutes, filtered, and the filter cake was dried to obtain the crude title compound 2a (18 g), which was directly used in the next reaction without purification.
[0272] MS m / z(ESI):285.9[M+1]
[0273] second step
[0274] 5-Bromo-4-hydrazino-6-phenylpyrimidin-2-amine 2b
[0275] Crude compound 2a (17.99 g, 63.21 mmol) was dissolved in 120 mL of ethanol, 50 mL of 85% hydrazine hydrate was added, and the reaction was stirred for 17 hours. Stop the reaction, filter the reaction solution, wash the filter cake with ethanol (30mL×2) and n-hexan...
Embodiment 3
[0291] 2-Ethyl-8-(2-methyl-6-(trifluoromethyl)pyridin-4-yl)-7-phenyl-[1,2,4]triazolo[1,5-c] Pyrimidin-5-amine 3
[0292]
[0293]
[0294] first step
[0295] 8-Bromo-3-ethyl-7-phenyl-[1,2,4]triazolo[4,3-c]pyrimidin-5-amine 3a
[0296] Compound 2b (500 mg, 1.78 mmol) and (tri)ethyl orthopropionate (378 mg, 2.14 mmol) were dissolved in 20 mL of ethanol, and stirred for 2 hours under reflux. The reaction was stopped, cooled to room temperature, and the reaction liquid was concentrated under reduced pressure. The resulting residue was slurried with 5 mL of anhydrous ether for 0.5 hours, filtered, and the filter cake was dried to obtain the title compound 3a (495 mg, yield: 87.16%).
[0297] MS m / z(ESI):318.3[M+1]
[0298] second step
[0299] 2-Ethyl-8-(2-methyl-6-(trifluoromethyl)pyridin-4-yl)-7-phenyl-[1,2,4]triazolo[1,5-c] Pyrimidin-5-amine 3
[0300] Under a nitrogen atmosphere, compound 3a (150 mg, 0.471 mmol), compound 1b (189 mg, 0.66 mmol), [1,1'-bis(diphenylp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com