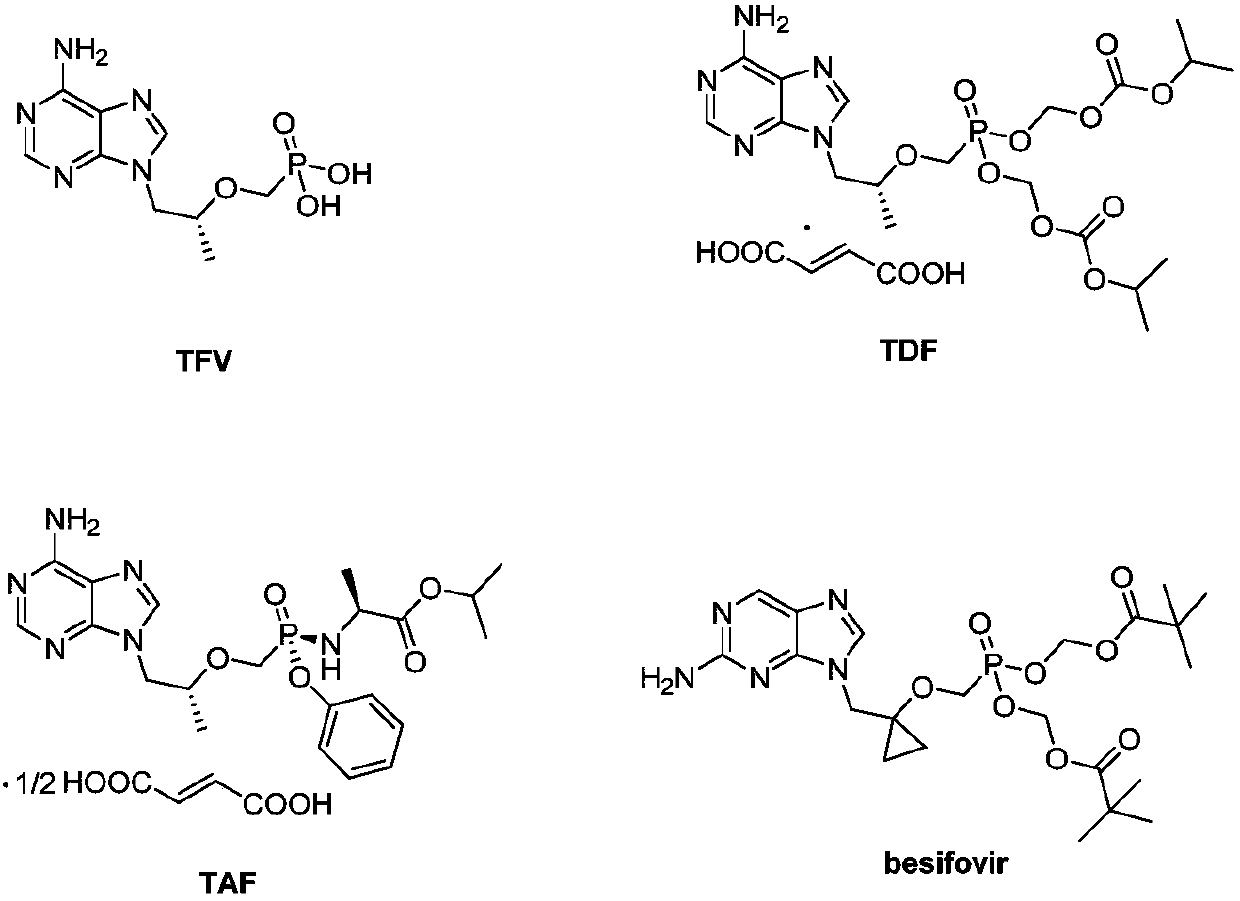
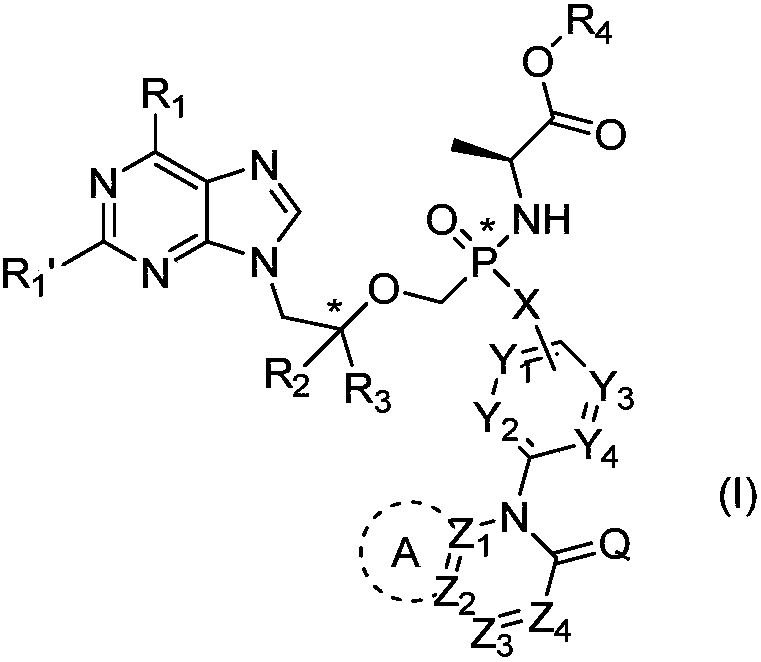
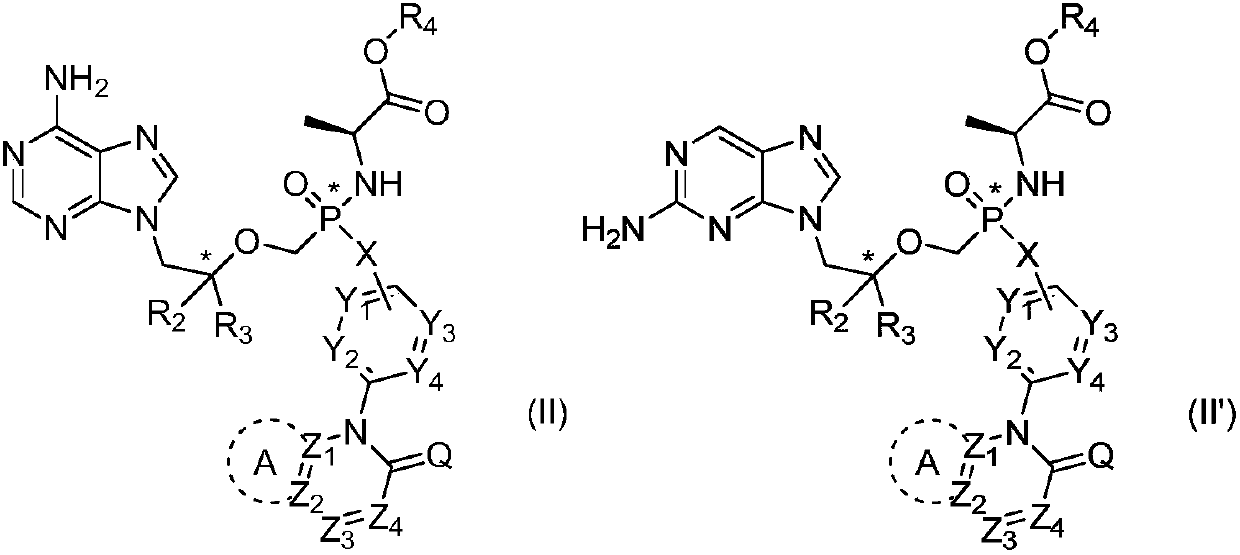
Phosphoramidate prodrug of nucleoside analogues, a pharmaceutical composition and applications of the phosphoramidate prodrug
A technology of nucleoside analogs and phosphoramidite esters, which can be used in drug combinations, compounds of Group 5/15 elements of the periodic table, medical preparations containing active ingredients, etc., and can solve problems such as lack of effective means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Embodiment 1. The preparation of intermediate 1a~1e
[0147]
[0148] Operation steps: Step 1: Dissolve p-bromophenol or substituted p-bromophenol (11.56mmol), p-toluenesulfonic acid (0.116mmol) in 15mL of dichloromethane solution, then add dropwise 3.2mL of 2,3-dihydropyridine Furan (34.68mmol), stirred and reacted at room temperature for 1 hour, washed with 5% sodium hydroxide solution and saturated brine respectively, and the organic phase was dried with anhydrous sodium sulfate and concentrated to obtain a white solid, which was directly used in the next reaction.
[0149] The second step: in DMF, add the product of previous step (6.87mmol), 5-picoline (4.58mmol), anhydrous potassium carbonate (5.50mmol) and CuI (0.55mmol), the mixture is heated to 140 ℃ of reaction 5 hours, cooled to room temperature, filtered and collected the filtrate, evaporated to remove DMF, then dissolved in ethyl acetate, washed with water and saturated brine respectively, added anhydrous...
Embodiment 2
[0156] Embodiment 2. Preparation of intermediate 1f
[0157]
[0158] Operation steps: Step 1: Add 4-bromo-2-chloro-phenol (11.56mmol), potassium carbonate (17.34mmol), benzyl chloride (13.87mmol) into 15mL DMF, react at room temperature for 24 hours, filter, add 200mL of filtrate Ethyl acetate was then washed with 5% sodium hydroxide solution, water, and saturated brine, and the organic phase was dried by adding anhydrous sodium sulfate and concentrated to obtain a yellow concentrate, which was directly used in the next reaction.
[0159] Second step: in DMF, add the product (6.87mmol) of last step, 5-picoline (4.58mmol), cesium carbonate (5.50mmol) and CuI (0.55mmol), the mixture is heated to 140 ℃ of reaction 10 hours, Cool to room temperature, collect the filtrate after filtration, remove DMF by subtractive evaporation, then dissolve in ethyl acetate, wash with water and saturated brine respectively, add anhydrous sodium sulfate to the organic phase, dry and concentrate...
Embodiment 3
[0161] Embodiment 3. Preparation of Intermediates 2a~2c
[0162]
[0163] Operation steps: Step 1: Dissolve p-bromophenol (11.56mmol) and p-toluenesulfonic acid (0.116mmol) in 15mL of dichloromethane solution, then add 3.2mL of 2,3-dihydropyran (34.68mmol) dropwise, After stirring and reacting at room temperature for 1 hour, the mixture was washed with 5% sodium hydroxide solution and saturated brine, and the organic phase was dried over anhydrous sodium sulfate and concentrated to obtain a white solid, which was directly used in the next reaction.
[0164] The second step: add the product of the previous step (6.87mmol), pyridone or substituted pyridone (4.58mmol), anhydrous potassium carbonate (5.50mmol) and CuI (0.55mmol) in DMF, and the mixture is heated to 140 ° C for reaction After 5 hours, cool to room temperature, collect the filtrate after filtration, remove DMF by subtractive evaporation, then dissolve in ethyl acetate, wash with water and saturated brine respecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com