Engineering-transformed macrophages and application of macrophages to septicopyemia
A technology of macrophages and inflammation, applied in the field of biomedicine, can solve the problems of difficult anti-inflammatory effects, short effective time of about 2-10 minutes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
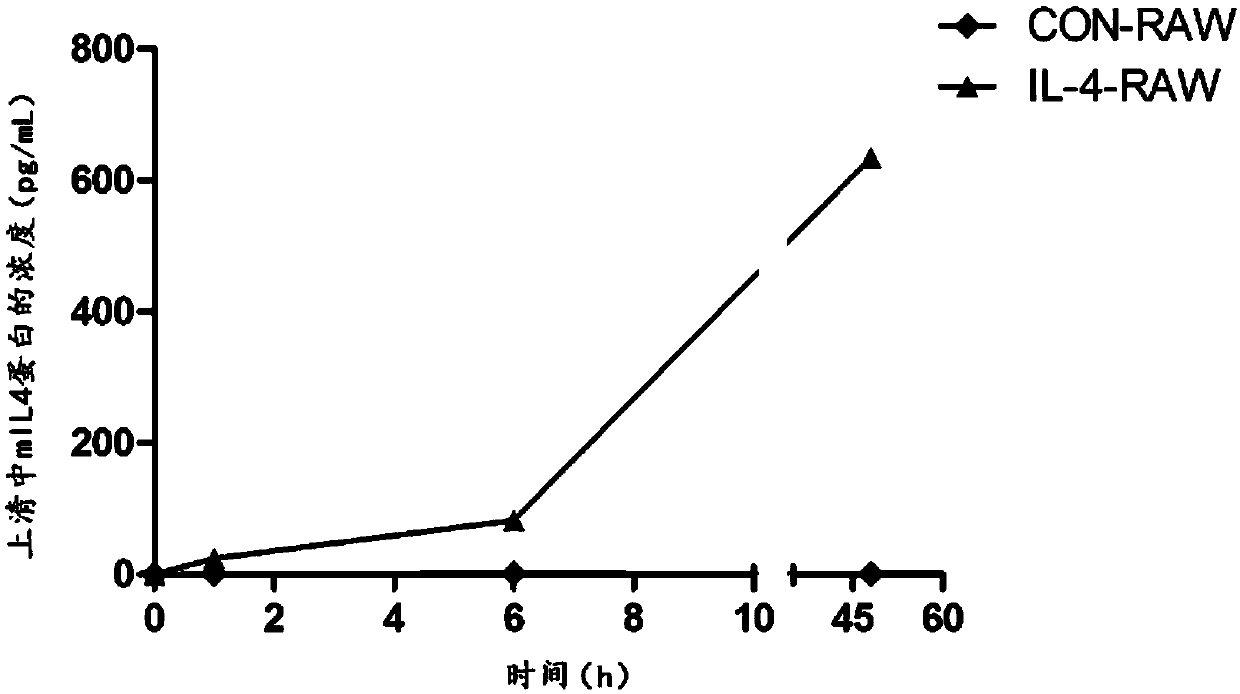
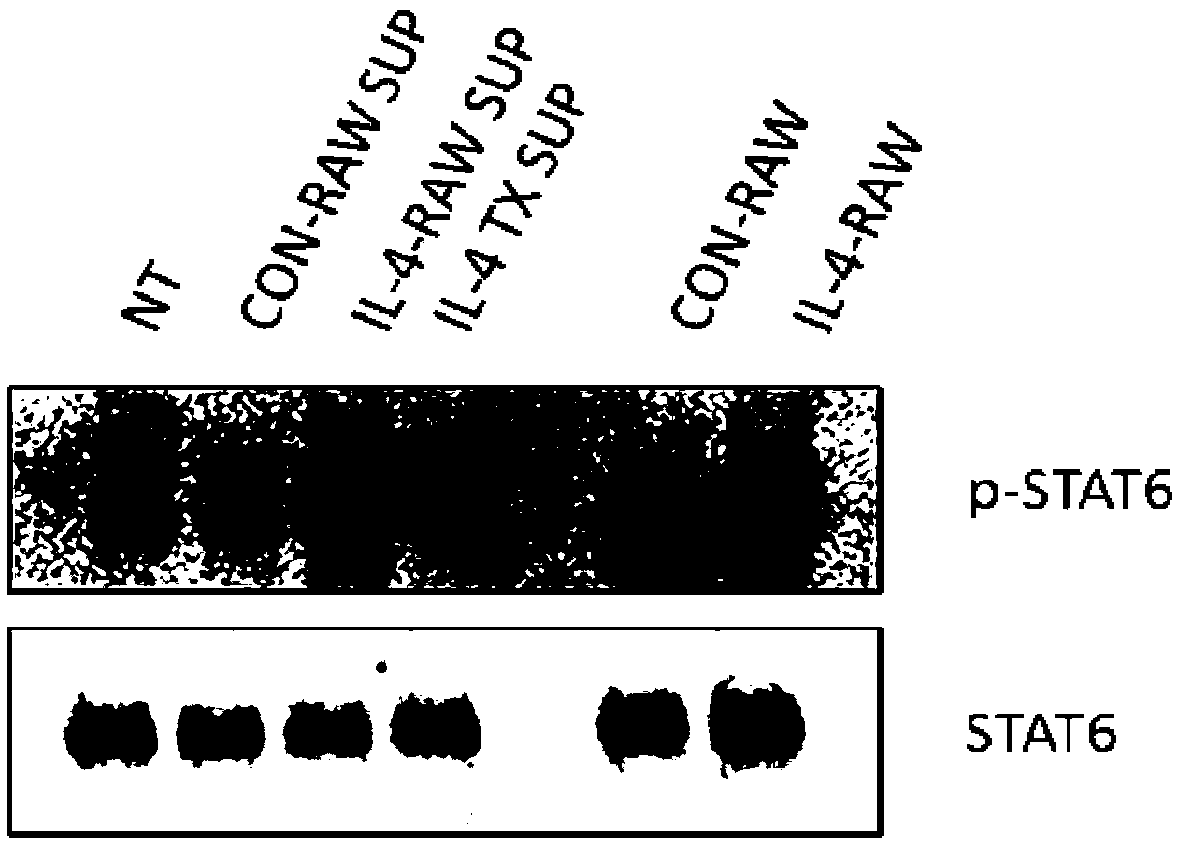
[0051] Example 1. Acquisition of a macrophage cell line stably expressing mIL4 protein (i.e. RAW264.7 cells or IL-4-RAW cells infected with lentivirus pCDH-mIL4) and detection of its secreted mIL4 protein
[0052]1. Construction of recombinant plasmid pCDH-mIL4
[0053] Insert the mIL4 gene between the recognition site of the restriction endonuclease EcoRI and the BamHI recognition site of the vector pCDH-MSCV-MCS-EF1α-copGFP-T2A-Puro (the nucleotide sequence is shown in sequence 1 in the sequence listing) , to obtain the recombinant plasmid pCDH-mIL4.
[0054] The recombinant plasmid pCDH-mIL4 expresses mIL4 protein, and its amino acid sequence is shown in sequence 2 in the sequence listing.
[0055] 2. Obtaining the lentivirus pCDH-mIL4 concentrate
[0056] 1. Inoculate the 293T cells grown to the logarithmic growth phase in a petri dish (60 mm in diameter) filled with 10 mL of conventional medium, at 37 °C, 5% CO 2 nourish.
[0057] 2. Gently mix 1 μg pVSVg, 3 μg psPAX2...
Embodiment 2
[0089] Embodiment 2, the application of IL-4-RAW cell
[0090] C57BL / 6 wild-type mice are products of Beijing Huafukang Biotechnology Co., Ltd.
[0091] PBS buffer is pH7.2-7.4, 67mM PO 4 (Ca and Mg free) PBS buffer.
[0092]1. Take 24 C57BL / 6 wild-type mice at 6-8 weeks, and inject LPS intraperitoneally (the mouse sepsis model is prepared by intraperitoneally injecting LPS), and the injection dose is 20 mg / kg.
[0093] 2. One hour after completing step 1, 24 C57BL / 6 wild-type mice were randomly divided into three groups (8 mice in each group) of PBS group, CON-RAW group and IL-4-RAW group. Then proceed as follows:
[0094] PBS group (negative control group): each mouse was injected with 200 μL PBS buffer solution into the tail vein.
[0095] CON-RAW group (negative control group): Each mouse was injected with 200 μL of CON-RAW cell suspension into the tail vein.
[0096] The preparation method of CON-RAW cell suspension is as follows: collect CON-RAW cells, and then resu...
Embodiment 3
[0103] Example 3, IL-4-RAW cells release interleukin-4 for a long time
[0104] Female healthy C57BL / 6J mice are products of Beijing Huafukang Biotechnology Co., Ltd.
[0105] PBS buffer is pH7.2-7.4, 67mM PO 4 (Ca and Mg free) PBS buffer.
[0106] 1. Take 12 female healthy C57BL / 6J mice of 7-8 weeks, inject ketamine (for anesthesia) into each mouse intraperitoneally, and the injection dose is 220 mg / kg; On the stage, the neck hair was cut off, disinfected, and the skin of the neck was cut through a longitudinal incision to fully expose the trachea. A syringe was inserted into the trachea between the two tracheal cartilage rings, and LPS solution was instilled at a dose of 15 mg / kg.
[0107] 2. After completing the step 10.5 hours, 12 female healthy C57BL / 6J mice were randomly divided into three groups (4 mice in each group) of PBS group, IL-4 group and IL-4-RAW group. Then proceed as follows:
[0108] PBS group (negative control group): 25 μL of PBS buffer was injected in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com