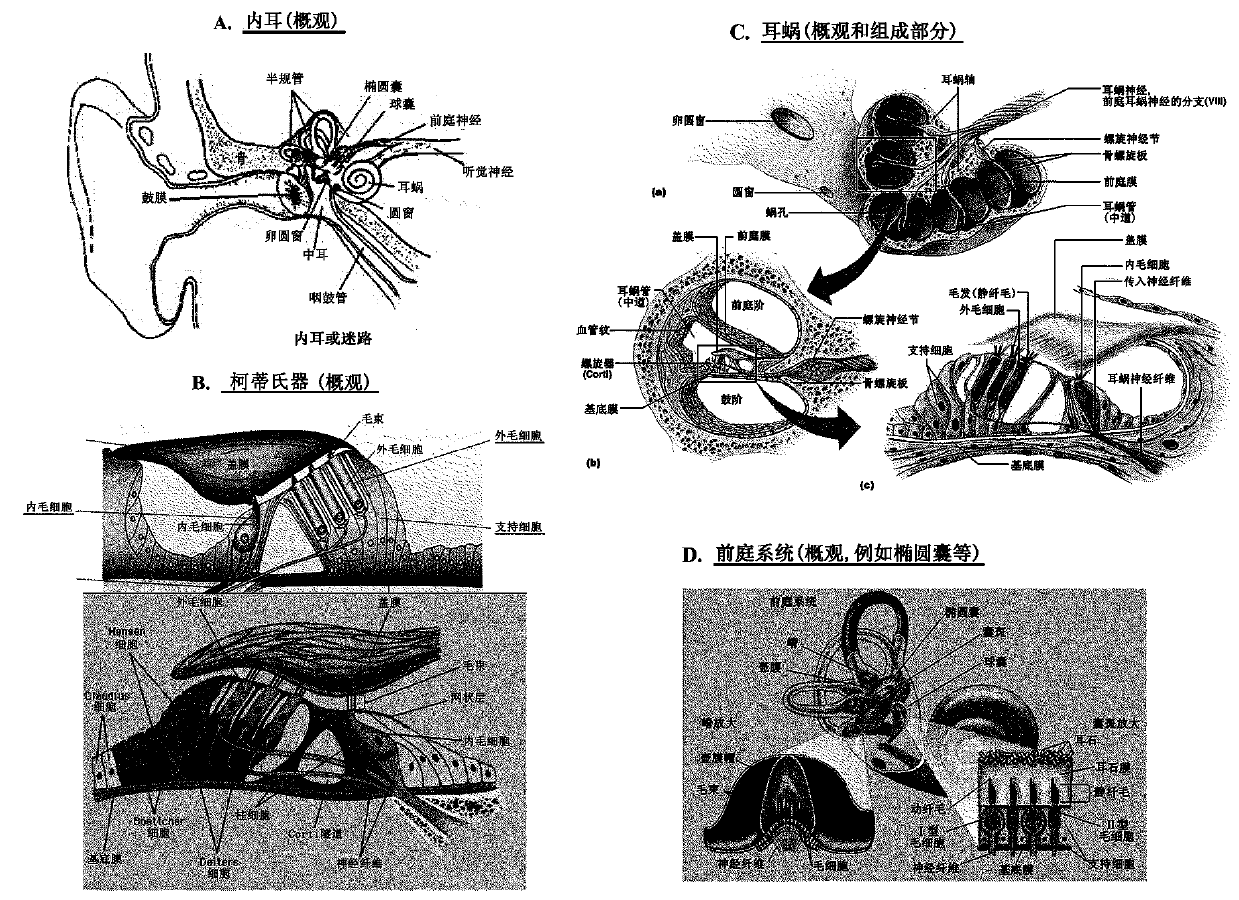
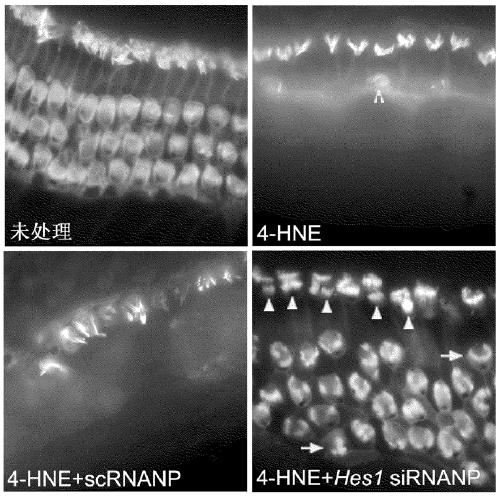
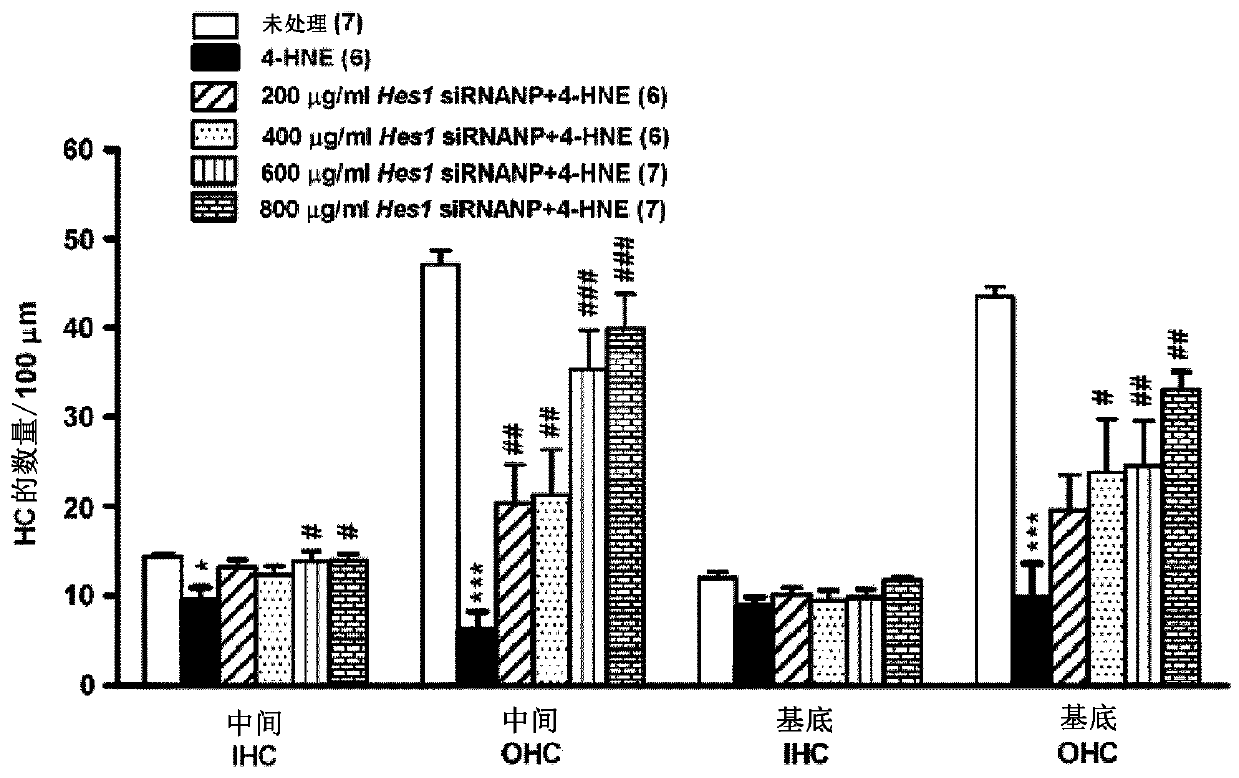
Combination therapies for inner ear sensory hair cell regeneration/replacement
A composition and biodegradation technology, applied in sensory diseases, animal cells, nervous system cells, etc., can solve problems such as unclear effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Example 1 - Generation of siHes1 nanoparticles and their evaluation
[0141] generate loaded nanoparticles
[0142] siRNA-loaded PLGA nanoparticles prepared for this study were previously reported (Cun et al. (2010) Intl.J.Pharmaceutics 390:70-75; Du et al. (2013) Hear.Res.304C:91-110 ) of water-in-oil-in-water (wl / o / w2) double emulsion solvent evaporation method to prepare. Briefly, siRNA was dissolved in 50 μL of TE buffer (10 mM Tris-HCl and 1 mM EDTA in MilliQ water, pH 7.5) and mixed with 100 mL of dichloromethane (DCM) containing 100 mg PLGA, and the mixture was sonicated Emulsifies into a primary wl / o emulsion. 4 mL of 5% (w / v) polyvinyl alcohol (PVA) in MilliQ aqueous solution was directly poured into the primary emulsion, which was then further emulsified by sonication for 30 s × 3 to form a wl / o / w2 double emulsion. The resulting emulsion was diluted with 50 mL of 0.3% (w / v) PVA in MilliQ in water and stirred magnetically at room temperature for 2 hours ...
Embodiment 2
[0173] Example 2 - BIO and Hes1 siRNA
[0174] Undamaged PC
[0175] Organotypic cultures from murine cochleae were grown and then harvested from CD1 mice at postnatal day 3 (P3). These explants were then cultured in appropriate medium for 24 hours. At the equivalent of P4 (i.e., 24 h ex vivo), cultured Organs of Corti (OC, i.e. cochlear sensory epithelial cells) were immersed in DMSO (vehicle) or the GSK3 inhibitor 6-bromoindirubin-3 '-oxime (BIO) in fresh medium, and correspondingly cultured OC for 72 hours. A subset of cultures from both treatment groups (jetSI 10 mM, PolyPlusTransfection, Illkirch, France) were transfected with 20 nM Hes1 siRNA for 24 hours corresponding to P7 (ie 96 hours ex vivo). To examine the effect of sequential administration, siHes1 was transfected in medium without BIO. To examine the effect of simultaneous administration, siHes1 was transfected in BIO-containing medium. Following the 24 hour transfection culture period, sequentially trea...
Embodiment 3
[0179] Example 3 - TIDE and Hes1 siRNA
[0180] Analysis of Tideglusib
[0181] Tideglusib was purchased from Cayman Chemical Company and was subjected to dose curve analysis under serum starvation conditions in which dog kidney passage (Madin-Darby Canine Kidney, MDCK) cells were paralleled with a series of commercially available GSK3 inhibitors to determine its efficacy under mitotic arrest conditions. Relative proliferative potential in a mature mammalian epithelial cell line. In these experiments, MDCK cells were cultured for 24 hours under serum starvation conditions and then treated with a GSK3 inhibitor and 10 μM EdU (5-ethynyl-2'-deoxyuridine), a permanent marker of cells undergoing DNA replication. The cells were cultured for 48 hours in the presence of nucleoside analogs).
[0182] Figure 19 An example of this type of analysis is depicted in . Each of the GSK3 inhibitors tested exhibited a clear positive effect on cell proliferation at the lowest doses teste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com