Crystal form of 7, 8-dihydroxy flavone derivative and preparation method and application thereof
A technology of dihydroxyflavonoids and derivatives is applied in the directions of organic chemical methods, medical preparations containing active ingredients, and drug combinations, which can solve the problems of affecting the bioavailability of drugs and affecting the absorption of drugs, and achieve low hygroscopicity and preparation. Simple method and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of crystalline form D of 7,8-dihydroxyflavone derivatives as shown in formula I
[0068] Refer to 4-oxo-2phenyl-4H-chromene-7,8-diyl bis(dimethylcarbamate) compound R in patent CN201380062367.X 7 In the preparation of the raw material, dimethylcarbamoyl chloride is replaced by methylcarbamoyl chloride to obtain 7,8-dihydroxyflavone derivatives as shown in formula I.
[0069]
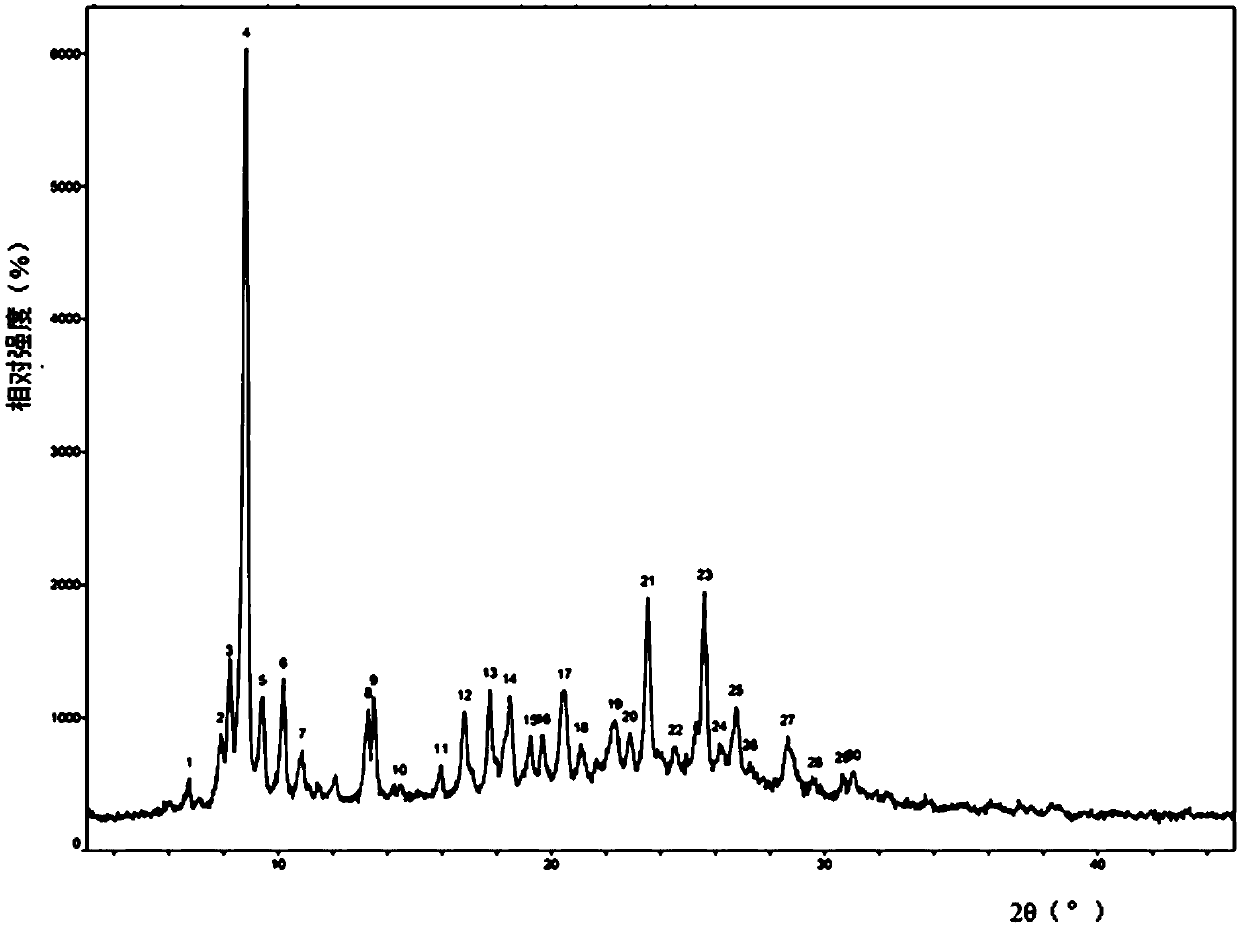
[0070] After XRPD detection, its X-ray powder diffraction pattern represented by 2θ angle is at 6.745±0.2°, 7.896±0.2°, 8.212±0.2°, 8.839±0.2°, 9.433±0.2°, 10.201±0.2°, 10.890± 0.2°, 13.300±0.2°, 13.500±0.2°, 14.432±0.2°, 15.961±0.2°, 16.814±0.2°, 17.742±0.2°, 18.472±0.2°, 19.224±0.2°, 19.692±0.2°, 20.484± 0.2°, 21.078±0.2°, 22.319±0.2°, 22.873±0.2°, 23.542±0.2°, 24.545±0.2°, 25.613±0.2°, 26.146±0.2°, 26.760±0.2°, 28.653±0.2°, 30.654± There are characteristic diffraction peaks at 0.2° and 31.061±0.2°; its XRPD pattern is as follows figure 1 shown.
Embodiment 2
[0071] Example 2 Preparation of crystalline form A of 7,8-dihydroxyflavone derivatives as shown in formula I
[0072] Weigh 200 mg of the 7,8-dihydroxyflavone derivative represented by formula I into an 8 mL glass bottle, add 4 mL of methanol, and shake for 2 minutes until the mixture is uniform. After stirring at 50°C for 1 day, the solution was in a suspension state, centrifuged and dried to obtain 182 mg of solid.
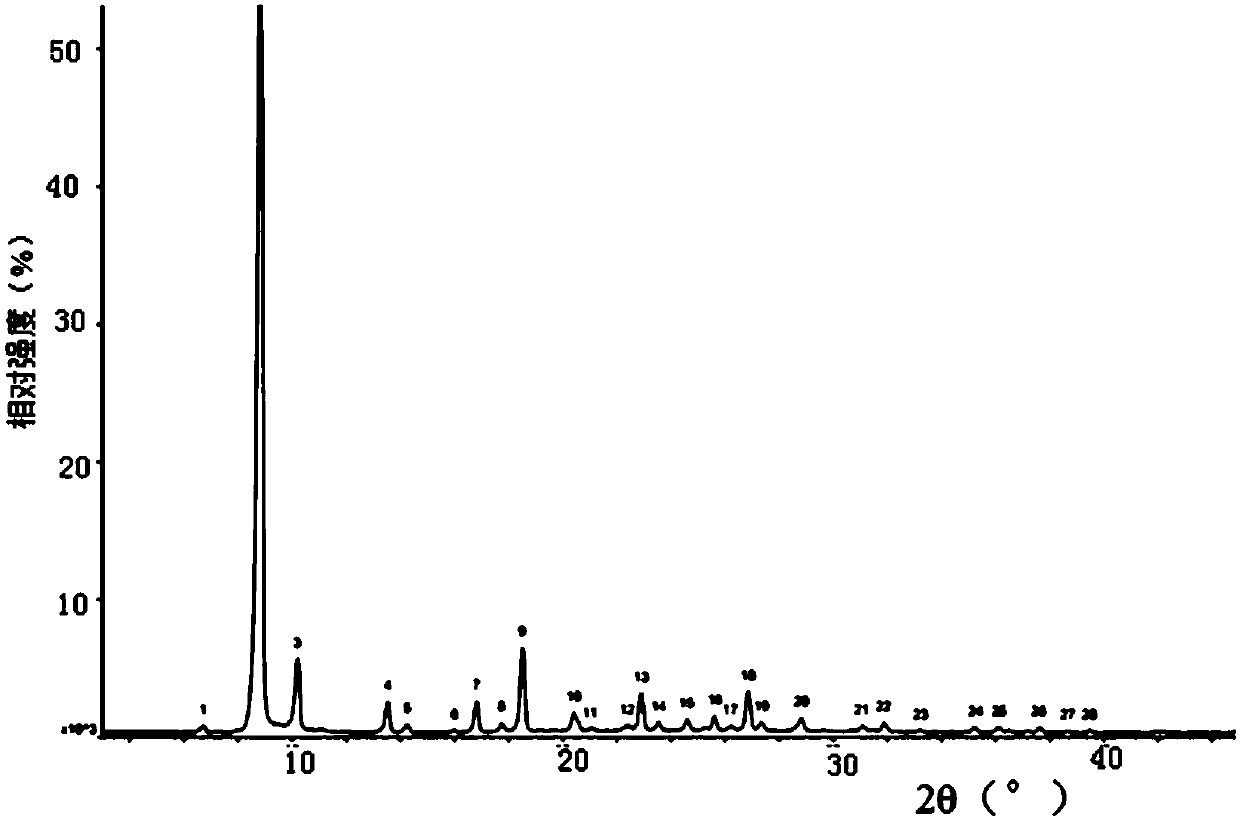
[0073] After XRPD detection, its X-ray powder diffraction pattern represented by 2θ angle is at 6.710±0.2°, 8.821±0.2°, 10.203±0.2°, 13.537±0.2°, 16.814±0.2°, 18.511±0.2°, 20.424± 0.2°, 22.910±0.2°, 25.631±0.2°, 26.857±0.2°, 31.064±0.2°, 33.229±0.2°, 35.260±0.2°, 36.131±0.2°, 37.594±0.2°, 38.678±0.2° and 39.470± There is a characteristic diffraction peak at 0.2°, and its XRPD pattern is as follows figure 2 shown.
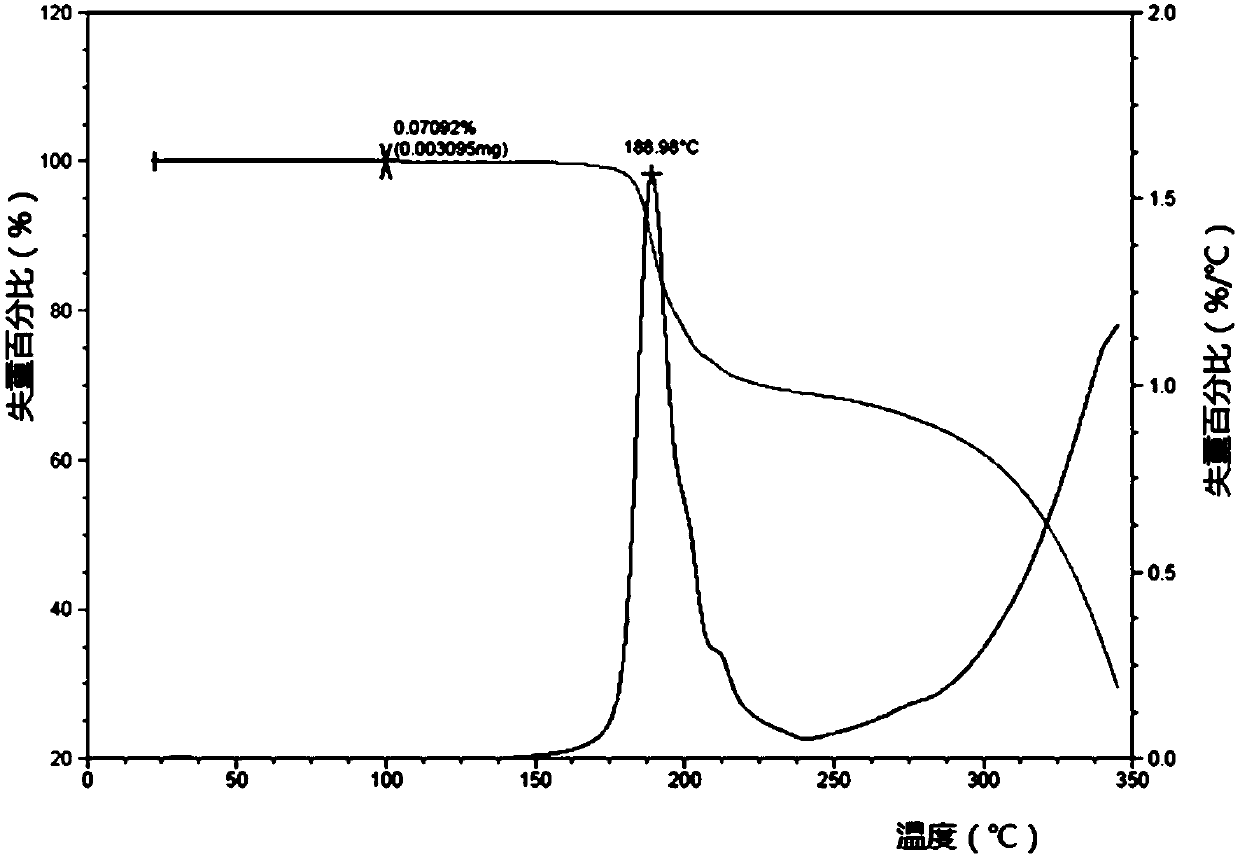
[0074] It is detected by TGA that the weight loss at 100±3°C accounts for 0.45% of the weight before weight loss. It is an ansolvate, and the wei...
Embodiment 3
[0077] Example 3 Preparation of Form A of 7,8-dihydroxyflavone derivatives as shown in Formula I
[0078] Weigh 500 mg of 7,8-dihydroxyflavone derivative shown in formula I into a 40 mL glass bottle, add 10 mL of ethanol, and mix well. After stirring at 50°C for 1 day, the solution was in a suspension state, centrifuged and dried to obtain 465 mg of solid. Its identification data are the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Heat of fusion | aaaaa | aaaaa |
| Heat of fusion | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com