Antitumor drug and preparation method and application thereof
An anti-tumor drug and drug technology, applied in anti-tumor drugs, drug combinations, organic chemistry and other directions, can solve the problems of poor targeting, complex structure, difficult synthesis, etc., to reduce production costs, the preparation method is simple and easy to operate, Achieve the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] preparation
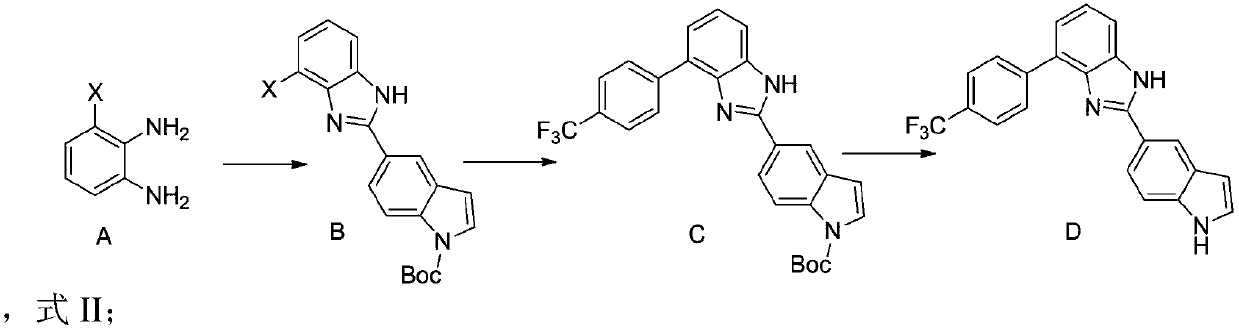
[0037] (1) Take 3-iodo-o-diamine (10mmol), N-tert-butoxycarbonyl-5-indole carboxaldehyde (11mmol), formic acid (12mmol), add to 20mL methanol, stir at 60°C for 5 hours to obtain Reaction solution: Add saturated aqueous sodium chloride solution and dichloromethane to the reaction solution for extraction, concentrate the organic phase and pass through column purification, collect the purified solution and distill under reduced pressure to obtain 4.0g (8.7mmol) N-tert-butoxycarbonyl-2 -(Acetamidomethyl)-3-bromopyrrole, the yield is 87%.
[0038] The hydrogen spectrum is as follows: 1 H NMR (400MHz, DMSO-d6) δ (ppm) = 1.63 (s, 9H), 5.85 (s, 1H), 6.38 (d, 1H), 6.99 (t, 1H), 7.46 (d, 1H), 7.59 (d,1H),7.65(d,1H),8.01(d,1H),8.15(d,1H),8.25(s,1H).
Embodiment 2
[0040] preparation
[0041] (1) take (5mmol), 4-trifluoromethylphenylboronic acid (5.5mmol), palladium acetate (0.1mmol), potassium acetate (6mmol), add 5mL dioxane and 5mL water, stir at 80°C for 10 hours to obtain the reaction liquid; the reaction solution was passed through diatomaceous earth, and the filtrate was collected; saturated aqueous sodium chloride solution and dichloromethane were added to the filtrate for extraction, and after the organic phase was concentrated, the column was purified, and the purified solution was distilled under reduced pressure to obtain 2.09g (4.4mmol) The yield was 88%.
[0042] The hydrogen spectrum is as follows: 1 H NMR (400MHz, DMSO-d6) δ (ppm) = 1 H NMR (400MHz, DMSO-d6) δ (ppm) = 1.63 (s, 9H), 5.87 (s, 1H), 6.56 (d, 1H), 7.35 (dd, 2H), 7.40 (d, 1H), 7.59 (d,1H), 7.65(d,1H), 7.69(dd,2H), 8.01(d,1H), 8.15(d,1H), 8.20(s,1H).
[0043] The inventor's initial synthetic route is (1) reacting 3-iodo o-phenylenediamine with 5-indole...
Embodiment 3
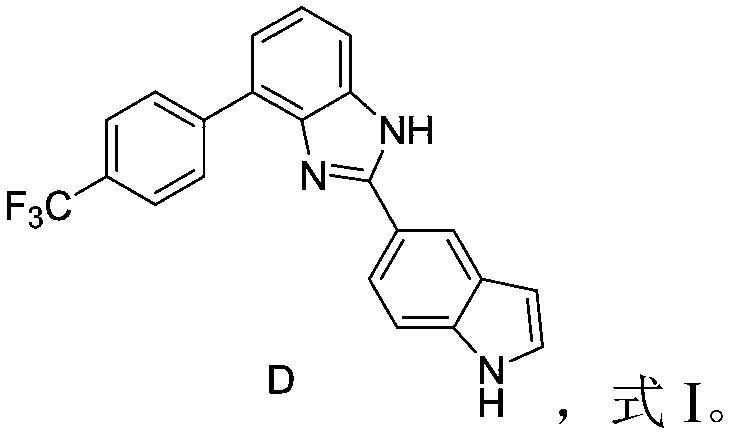
[0045] preparation That is, compound D shown in formula II:
[0046] (1) take (2mmol), was added to the hydrochloric acid-dioxane solution, stirred at 25°C for 3 hours to obtain a reaction solution; the reaction solution was distilled under reduced pressure to obtain 716mg (1.9mmol) The yield is 95%.
[0047] The hydrogen spectrum is as follows: 1 H NMR (400MHz, DMSO-d6) δ (ppm) = 5.85 (s, 1H), 6.53 (d, 1H), 7.38 (dd, 2H), 7.43 (d, 1H), 7.56 (d, 1H), 7.61 (d,1H), 7.68(dd,2H), 8.03(d,1H), 8.12(d,1H), 8.17(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com