Method for determining pitavastatin calcium content through HPLC method
A technology of pitavastatin calcium and pitavastatin, which is applied in the field of pitavastatin calcium determination, can solve the problems of no report on the determination of pitavastatin calcium content, and achieve the effects of low cost, high accuracy and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for measuring pitavastatin calcium content by HPLC, said method comprising the steps of:
[0031] S1. Prepare internal standard solution: add acetonitrile and water mixed solution to butylparaben, shake well to obtain internal standard solution;
[0032] S2, prepare reference substance solution: add the mixed solution of acetonitrile and water, internal standard solution successively to pitavastatin methylbenzylamine, shake well, obtain reference substance solution;
[0033] S3, preparation need testing solution: in pitavastatin calcium, add the mixed solution of acetonitrile and water, internal standard solution successively, shake up, get need testing solution;
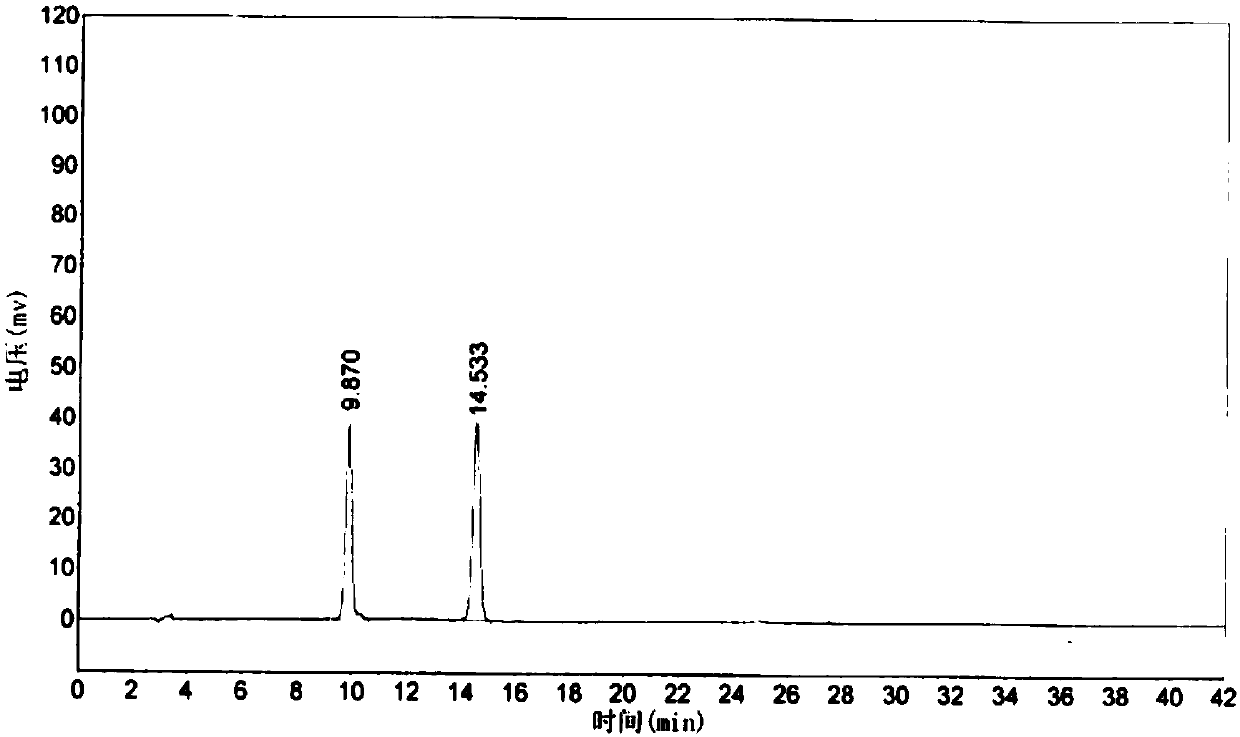
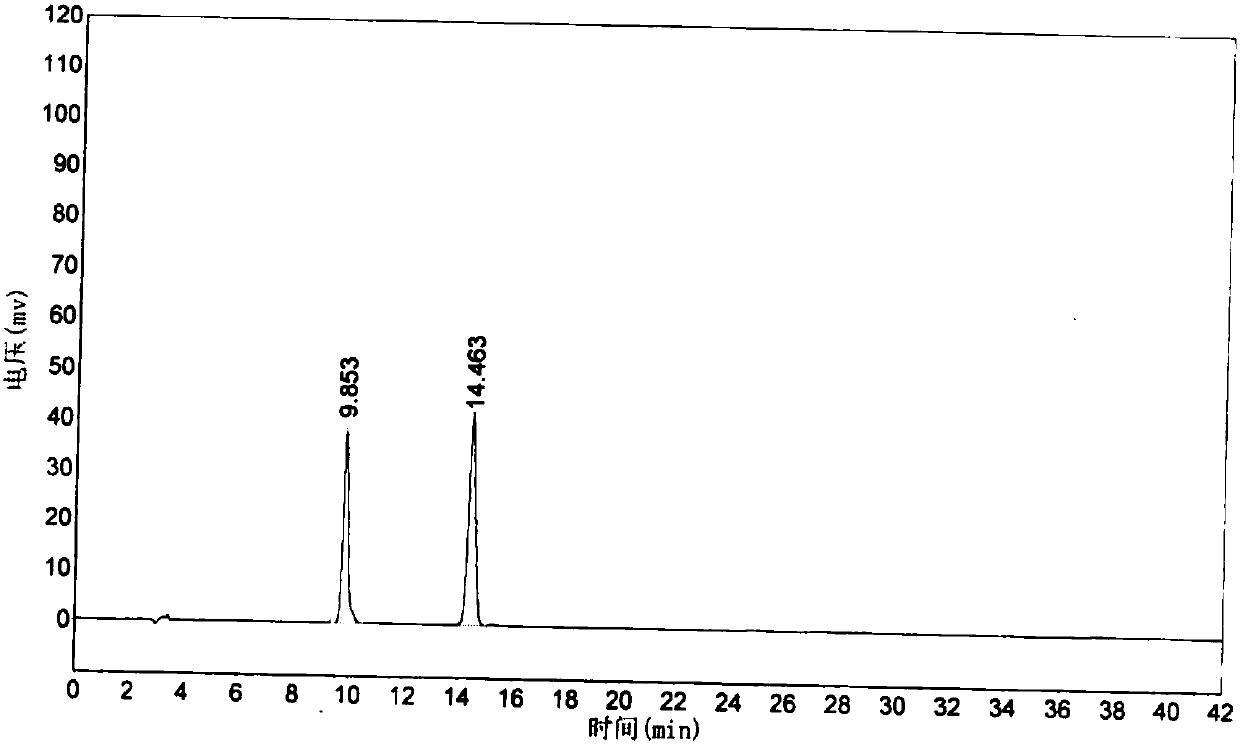
[0034] S4, chromatographic determination: take the reference substance solution, inject it into the HPLC instrument, and record the chromatogram Figure I ; Take the test solution, inject it into the HPLC instrument, and record the chromatogram Figure II , according to the color spectrum Figure I ...
Embodiment 2
[0036] A method for measuring pitavastatin calcium content by HPLC, said method comprising the steps of:
[0037] S1. Prepare internal standard solution: add acetonitrile and water mixed solution to butylparaben, shake well to obtain internal standard solution;
[0038] S2, prepare reference substance solution: add the mixed solution of acetonitrile and water, internal standard solution successively to pitavastatin methylbenzylamine, shake well, obtain reference substance solution;
[0039] S3, preparation need testing solution: in pitavastatin calcium, add the mixed solution of acetonitrile and water, internal standard solution successively, shake up, get need testing solution;
[0040] S4, chromatographic determination: take the reference substance solution, inject it into the HPLC instrument, and record the chromatogram Figure I ; Take the test solution, inject it into the HPLC instrument, and record the chromatogram Figure II , according to the color spectrum Figure I ...
Embodiment 3
[0043] A method for measuring pitavastatin calcium content by HPLC, said method comprising the steps of:
[0044] S1. Prepare internal standard solution: add acetonitrile and water mixed solution to butylparaben, shake well to obtain internal standard solution;
[0045] S2, prepare reference substance solution: add the mixed solution of acetonitrile and water, internal standard solution successively to pitavastatin methylbenzylamine, shake well, obtain reference substance solution;
[0046] S3, preparation need testing solution: in pitavastatin calcium, add the mixed solution of acetonitrile and water, internal standard solution successively, shake up, get need testing solution;
[0047] S4, chromatographic determination: take the reference substance solution, inject it into the HPLC instrument, and record the chromatogram Figure I ; Take the test solution, inject it into the HPLC instrument, and record the chromatogram Figure II , according to the color spectrum Figure I ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com