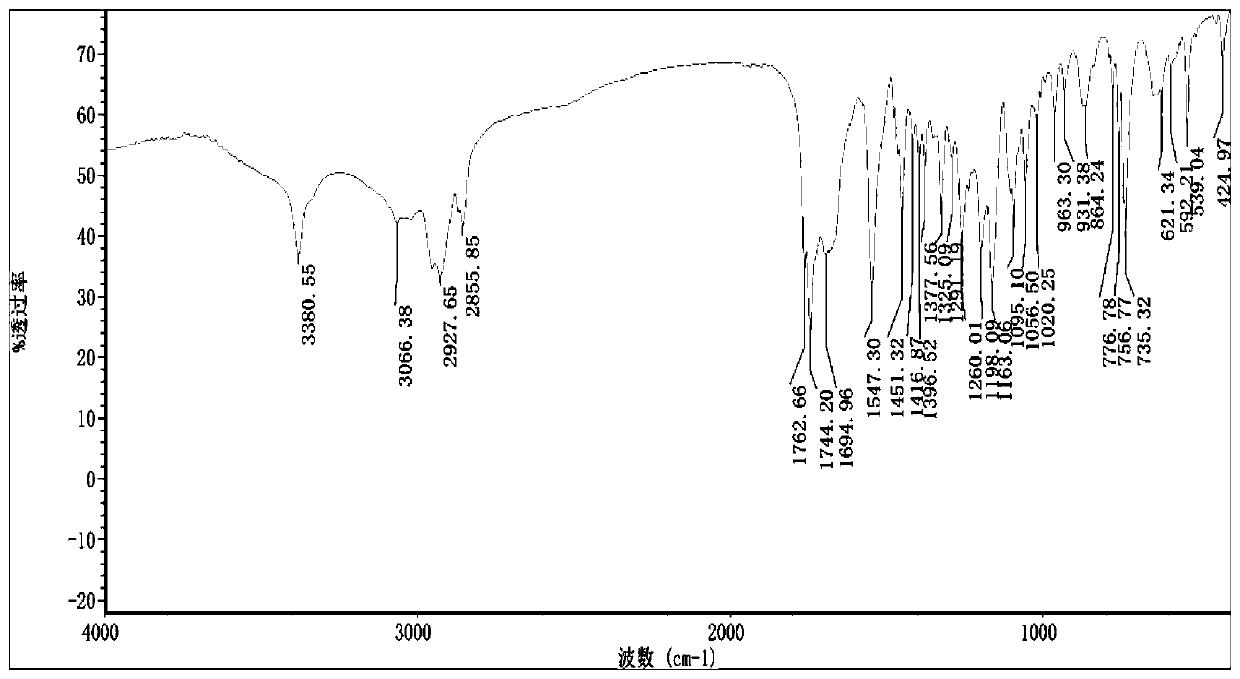
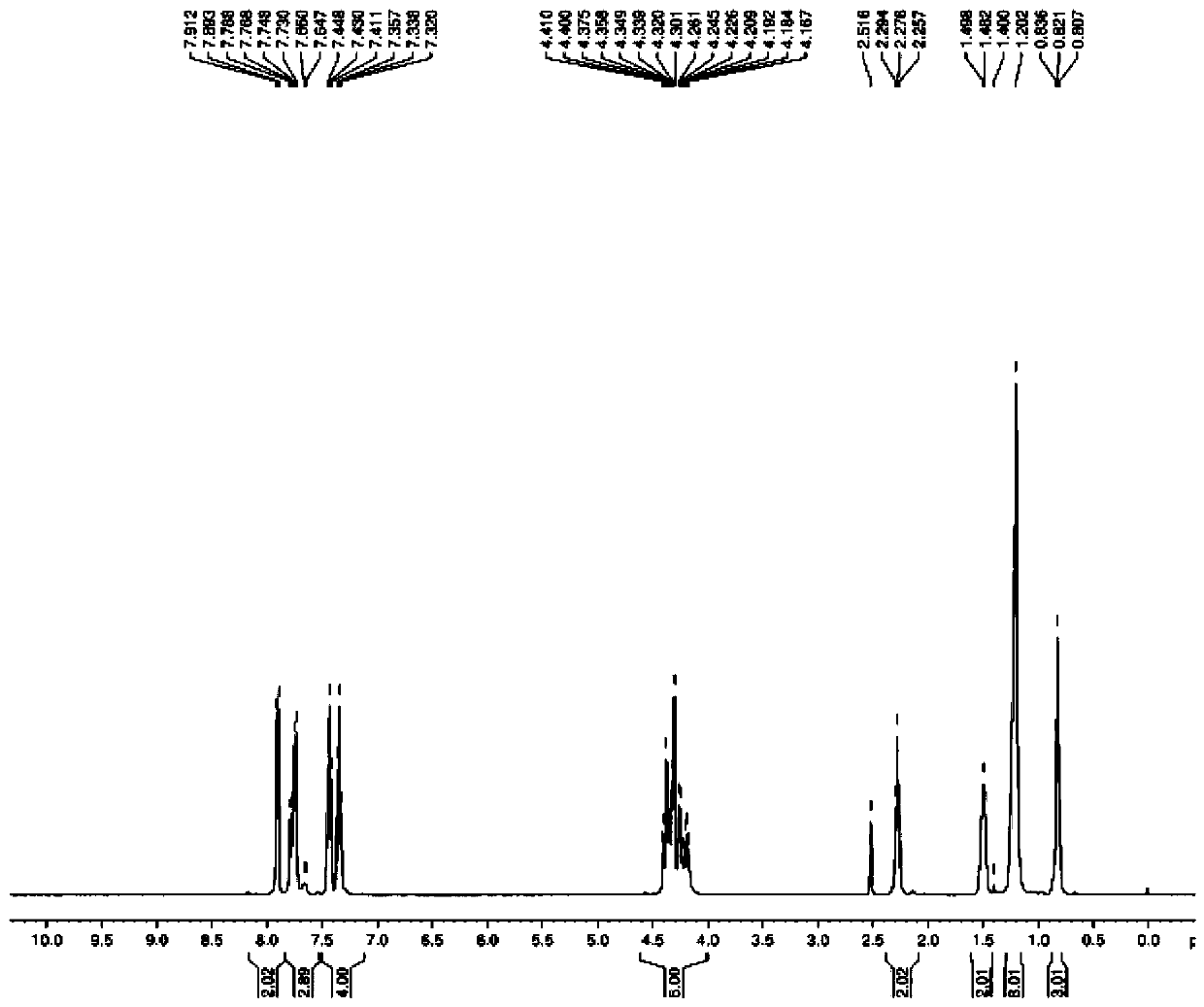
Preparation method of N-fmoc-D-methionine-O-octanoyl-serine/threonine
The technology of fluorene methoxycarbonyl and fluorene methoxycarbonyl- is applied in the field of preparation of serine/threonine derivatives, which can solve the problems of difficult operation, unenvironmental protection, and difficulty in purification, and achieve the effect of process stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Step 1: Dissolve 20g (0.048mol) of N-fluorenylmethoxycarbonyl-L-serine-benzyl ester in 260ml tetrahydrofuran until dissolved. Then add 11.2g (0.0776mol) of n-octanoic acid, cool down to 0 degrees Celsius in an ice-water bath, slowly add 9.78g (0.0776mol) of N,N'-diisopropylcarbodiimide dropwise, and drop N,N'- For diisopropylcarbodiimide, remove the ice-water bath, let it slowly rise to 25 degrees Celsius, and react overnight. The next day, after thin-layer chromatography analysis, the raw material N-fluorenylmethoxycarbonyl-L-serine-benzyl ester had completely reacted and could be processed. Treatment: Suction filter the liquid, slowly evaporate the tetrahydrofuran to dryness at 35 degrees Celsius to obtain an oily substance, dissolve the oily substance with 400ml ethyl acetate, wash the ethyl acetate phase twice with citric acid with a concentration of 5% by mass, After that, it was washed three times with water until pH = 7, washed once with brine, and dried over an...
Embodiment 2
[0015] Step 1: Dissolve 20g (0.048mol) of N-fluorenylmethoxycarbonyl-DL-serine-benzyl ester in 260ml tetrahydrofuran until dissolved. Then add 11.2g (0.0776mol) of n-octanoic acid, 1.2g (0.0098mol) of 1-hydroxybenzotriazole, cool down to 0 degrees Celsius in an ice-water bath, and add 15.8g of N,N'-dicyclohexylcarbodiimide in batches (0.0776mol), after adding N,N'-dicyclohexylcarbodiimide, remove the ice-water bath, let it slowly rise to 25 degrees Celsius, and react overnight. The next day, after thin-layer chromatography analysis, the raw material N-fluorenylmethoxycarbonyl-DL-serine-benzyl ester had completely reacted and could be processed. Treatment: Cool down to 0°C to obtain the liquid, evaporate the tetrahydrofuran to dryness at 35°C to obtain an oily substance, dissolve the oily substance with 400ml of ethyl acetate, wash the ethyl acetate phase twice with 5% citric acid by mass , washed twice with water until pH = 7, washed once with brine, and dried over anhydrous ...
Embodiment 3
[0018] Step 1: Dissolve 20g (0.048mol) of N-fluorenylmethoxycarbonyl-Dserine-benzyl ester in 260ml tetrahydrofuran until dissolved. Add 11.2 g (0.0776 mol) of n-octanoic acid, cool down to 0 degrees Celsius in an ice-water bath, add 14.7 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride in batches, and complete the addition 1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride, remove the ice-water bath, let it slowly rise to 25 degrees Celsius, and react overnight. The next day, after thin-layer chromatography analysis, the raw material N-fluorenylmethoxycarbonyl-D serine-benzyl ester had completely reacted and could be processed. Treatment: Suction filter the liquid, slowly evaporate the tetrahydrofuran to dryness at 35 degrees Celsius to obtain an oily substance, dissolve the oily substance with 400ml ethyl acetate, wash the ethyl acetate phase twice with citric acid with a concentration of 5% by mass, After that, it was washed three times with water unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com