Application of ertapenem disodium to preparation of medicine for preventing and treating bovine contagious rhinotracheitis
A technology for ertapenem sodium and rhinotracheitis, which is applied to the application field of ertapenem sodium in the preparation of medicines for preventing and treating bovine infectious rhinotracheitis, and can solve the problem of strong virulence, potential safety hazards, inability to remove viruses, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 virus-TCID 50 Determination of
[0042] MDBK cells (preserved by Dairy Cow Research Center, Shandong Academy of Agricultural Sciences) were digested and mixed with 1 × 10 per well. 5 Cells / mL were seeded into 96-well cell culture plates, placed in 37°C, 5% CO 2 After being cultured into a single layer of cells in the cell incubator, the cell growth solution in the well was discarded, and the virus dilution solution of bovine infectious rhinotracheitis virus (preserved by the Dairy Cow Research Center, Shandong Academy of Agricultural Sciences) was serially diluted 10 times (dilutions were respectively for 10 -1 ~10 -10 ) inoculated in a 96-well plate full of monolayer cells, 100 μL per well, placed in 37°C, 5% CO 2 Continue culturing in an incubator, observe the cytopathic effect (CPE) of the cells daily, and record the number of cytopathic wells in detail. At the same time, a normal cell control group and a blank control group were set up, with 8 repli...
Embodiment 2
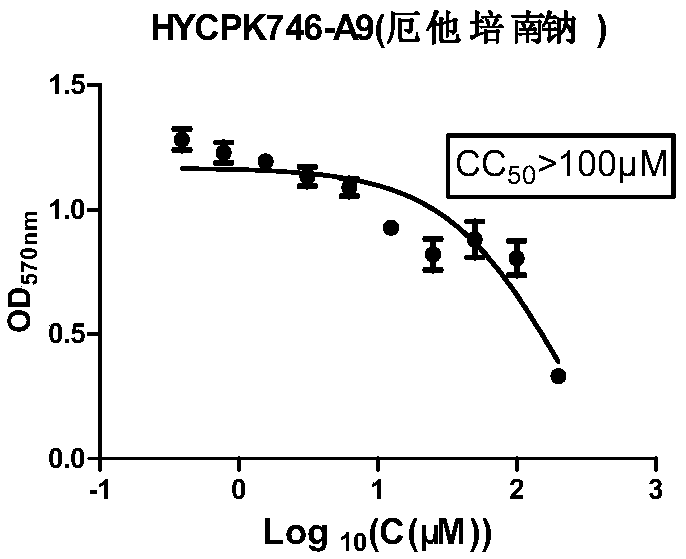
[0052] Example 2 Toxicity test of ertapenem sodium on MDBK cells:
[0053] MDBK cells are susceptible cells to bovine infectious rhinotracheitis virus. Therefore, the cytotoxicity of ertapenem sodium to MDBK cells was first detected, and the specific experimental steps were as follows:
[0054] (1) Inoculate 100 μL cells (MDBK 5000 cells / well) in a 96-well plate.
[0055] (2) After culturing for about 12 hours, the next step of drug addition analysis was carried out. The medium was discarded, and 100 μL of 2% FBS DMEM containing different drug concentrations (starting at 50 μM, serially diluted twice to obtain 9 concentrations of 25 μM, 12.5 μM, 6.25 μM, and 3.125 μM) was added to each well. Do 3 parallels for each concentration. At the same time, control wells: add 100 μL of 2% FBS DMEM medium containing 0.9% DMSO. Zero well: no cells are plated.
[0056] (3) At 37°C, 5% CO 2 After culturing for 48 h under the condition, the medium in the well was discarded. Wash twi...
Embodiment 3
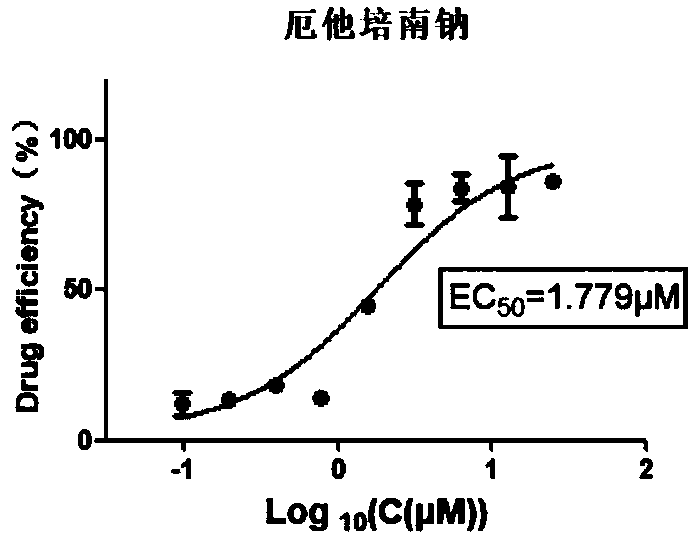
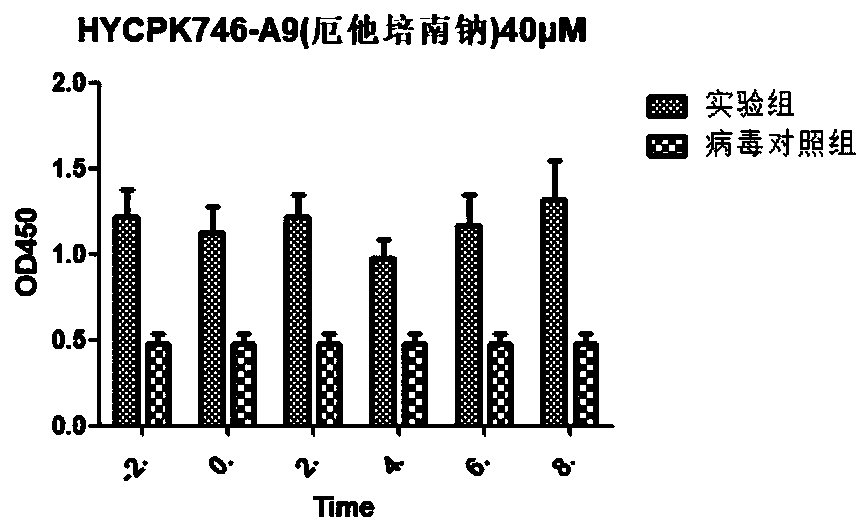
[0061] Example 3 Inhibition experiment of ertapenem sodium on bovine infectious rhinotracheitis virus:
[0062] (1) Inoculate 1×10 in each well of a 96-well plate 4 MDBK cells, 37°C, 5% CO 2 Cultivate overnight in an incubator;
[0063] (2) Discard the medium and add 100 μL 100TCID to each well 50 Bovine infectious rhinotracheitis virus virus dilution (use 2% FBS DMEM cells to overgrow and add virus dilution, according to 50 μ M initial concentration, two-fold concentration gradient dilution adds medicine, 5% CO 2 Cultivated in an incubator;
[0064] (3) After 48 hours, operate according to the instructions of the CCK-8 kit, and measure the OD value at 450 nm with a microplate reader.
[0065] (4) analysis data, virus inhibition rate (%)=(drug treatment group D450nm value-virus control group D450nm value) / (normal cell control group D450nm value-virus control group D450nm value) * 100%, get with GraphPad Prism5 software The half effective concentration of the compound (E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ec50 | aaaaa | aaaaa |
| Ec50 | aaaaa | aaaaa |
| Ec50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com