Quasi-peptide compound containing serine and preparation method and application thereof
A compound, serine technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combinations, etc., can solve problems such as adverse reactions and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
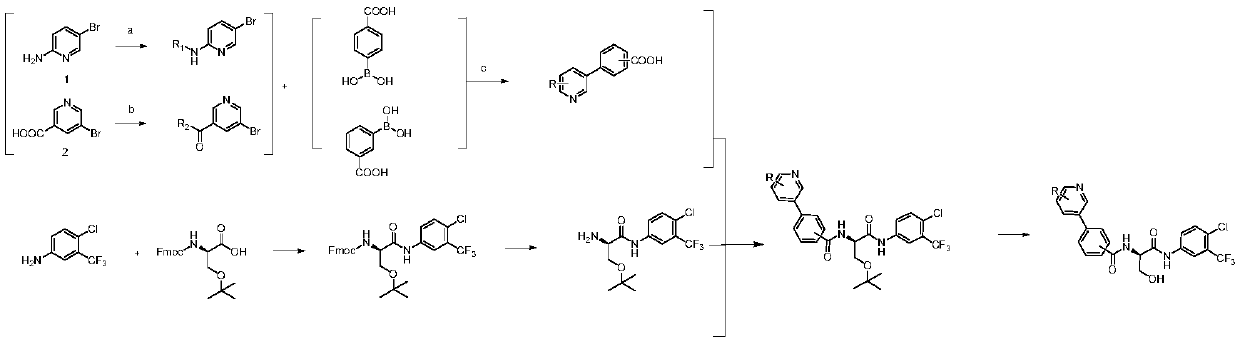
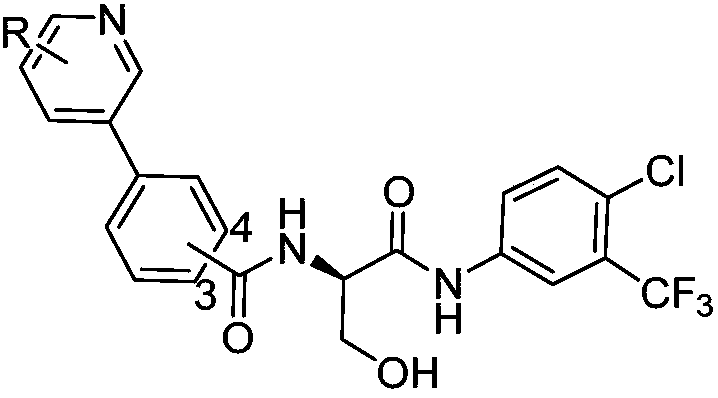
[0036] see figure 1 , the preparation method of the serine-containing peptoid compound of above-mentioned structure, comprises the following steps:
[0037] 1) Acylation of 5-bromo-2-aminopyridine with the corresponding acid chloride compound to prepare acylated 5-bromo-2-aminopyridine;
[0038] The specific process of the described step 1) is: dissolving 5-bromo-2-aminopyridine in anhydrous dichloromethane, adding triethylamine, and slowly adding the corresponding acid chloride compound dropwise to the above solution under ice-bath conditions After the dropwise addition was completed, the ice bath was removed and the reaction was carried out at room temperature for 12 hours. After the reaction, add dichloromethane to dilute, wash with water, wash with saturated sodium bicarbonate, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, distill under reduced pressure, and separate by column chromatography to obtain a white solid, which is the acylated 5- Brom...
Embodiment 1
[0054] A serine-containing peptoid compound, R is , the preparation method is as follows:
[0055] 1) Synthesis of N-(5-bromopyridin-2-yl)acetamide: 5-bromo-2-aminopyridine (5.19 g, 30 mmol) was dissolved in 100 ml of anhydrous dichloromethane, and 20 ml of triethylamine was added. Under the condition of ice bath, acetyl chloride (2.54ml) was slowly added dropwise to the above solution, after the dropwise addition was completed, the ice bath was removed, and the reaction was raised to room temperature overnight (ie 12h). After the reaction was finished, dichloromethane was added for dilution, washed with water (30ml×3), saturated NaHCO 3 Solution washing (30ml×3), saturated NaCl washing (30ml), organic phase anhydrous NaCl 2 SO 4 dry. Column chromatography separated 5.65 g of white solid with a yield of 88%. Mp78-81℃; EI-MS(m / z):214[M] + .
[0056] 2) Synthesis of 4-(6-(acetylamino)pyridin-3-yl)benzoic acid: N-(5-bromopyridin-2-yl)acetamide (4.30g, 20mmol), p-carboxyph...
Embodiment 2
[0064] A peptoid compound containing serine, R as The preparation method is as follows:
[0065] 1) in N 2 Under protection, add thionyl chloride (36ml, 494mmol) dropwise to 5-bromonicotinic acid (5.00g, 24.7mmol). After the dropwise addition, heat to reflux for 2-3h until the solution is clear, and spin off the chloride under reduced pressure. Sulfoxide, a light yellow solid was obtained. The solid was dissolved in 30ml of anhydrous dichloromethane, and the reactive intermediate solution was slowly added dropwise to a solution of cyclopropylamine (3.77ml) in dichloromethane (30ml) in an ice bath. After the dropwise addition, it was raised to room temperature and reacted overnight. After the reaction, add 2mol / L K to the reaction system 2 CO 3 Solution 20ml. Separate the liquid to take the dichloromethane phase, extract the aqueous phase with dichloromethane (15ml × 3), combine the organic phases, anhydrous Na 2 SO 4 dry. Column chromatography separation and purifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com