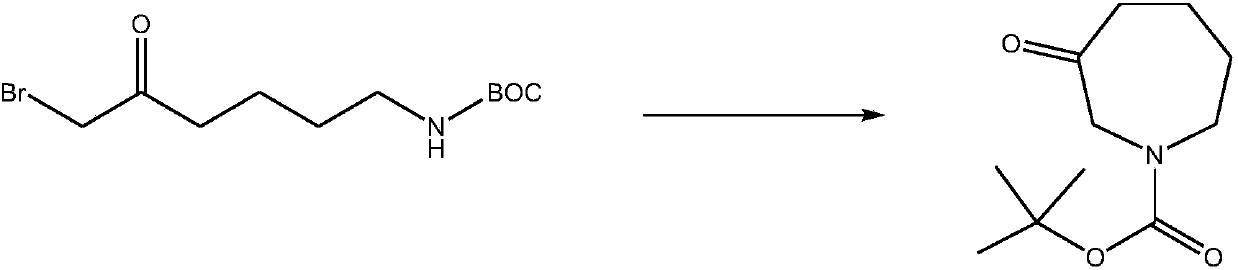Simple synthetic process of antidepressant medicine imipramine hydrochloride intermediate
A technique for the synthesis of imipramine hydrochloride, which is applied in organic chemistry and other fields, can solve the problems of expensive raw materials, few synthesis reports, difficult preparation and industrialization, and achieve the effects of easy-to-obtain raw materials, simple synthesis process, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The present invention will be further illustrated below in conjunction with specific embodiments, and it should be understood that the following specific embodiments are only used to illustrate the present invention and are not intended to limit the scope of the present invention.
[0015] The present invention boldly innovates on the basis of existing related techniques, and develops a technique with simple and easy raw materials and simple and convenient operation.
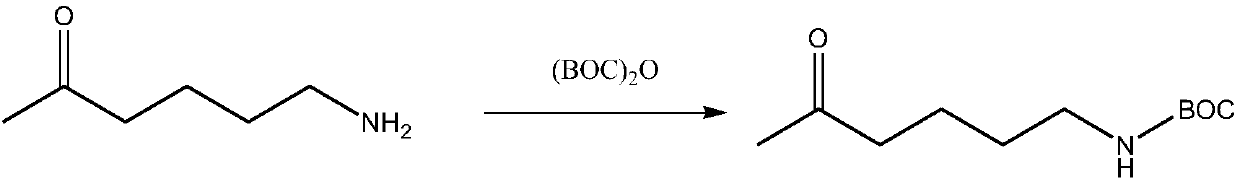
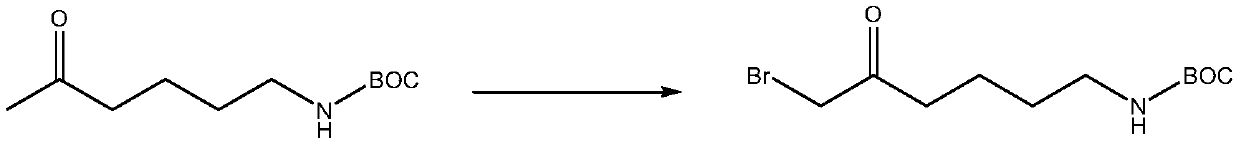
[0016] Its synthetic reaction formula is as follows:
[0017]
[0018] The specific steps are:
[0019] first step
[0020] Take 115g of 6-amino-2-hexanone, 240g of BOC anhydride, and 1000ml of DCM, put them into a 2L reaction flask, stir and react at room temperature 15-25°C overnight, about 16h; GC, until the raw materials are completely reacted. The concentrated solution obtained is directly thrown into the next step.
[0021] second step
[0022] Add 1g of AIBN and 196g of NBS to the concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com