Method for preparation of 5-hydroxymethylfurfural catalyzed by methyltrioxorhenium
A technology of methyl rhenium trioxide and hydroxymethylfurfural, which is applied in organic chemistry and other fields, can solve problems not involved in catalytic dehydration of six-carbon sugars, and achieve high selectivity, improved conversion rate and selectivity, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
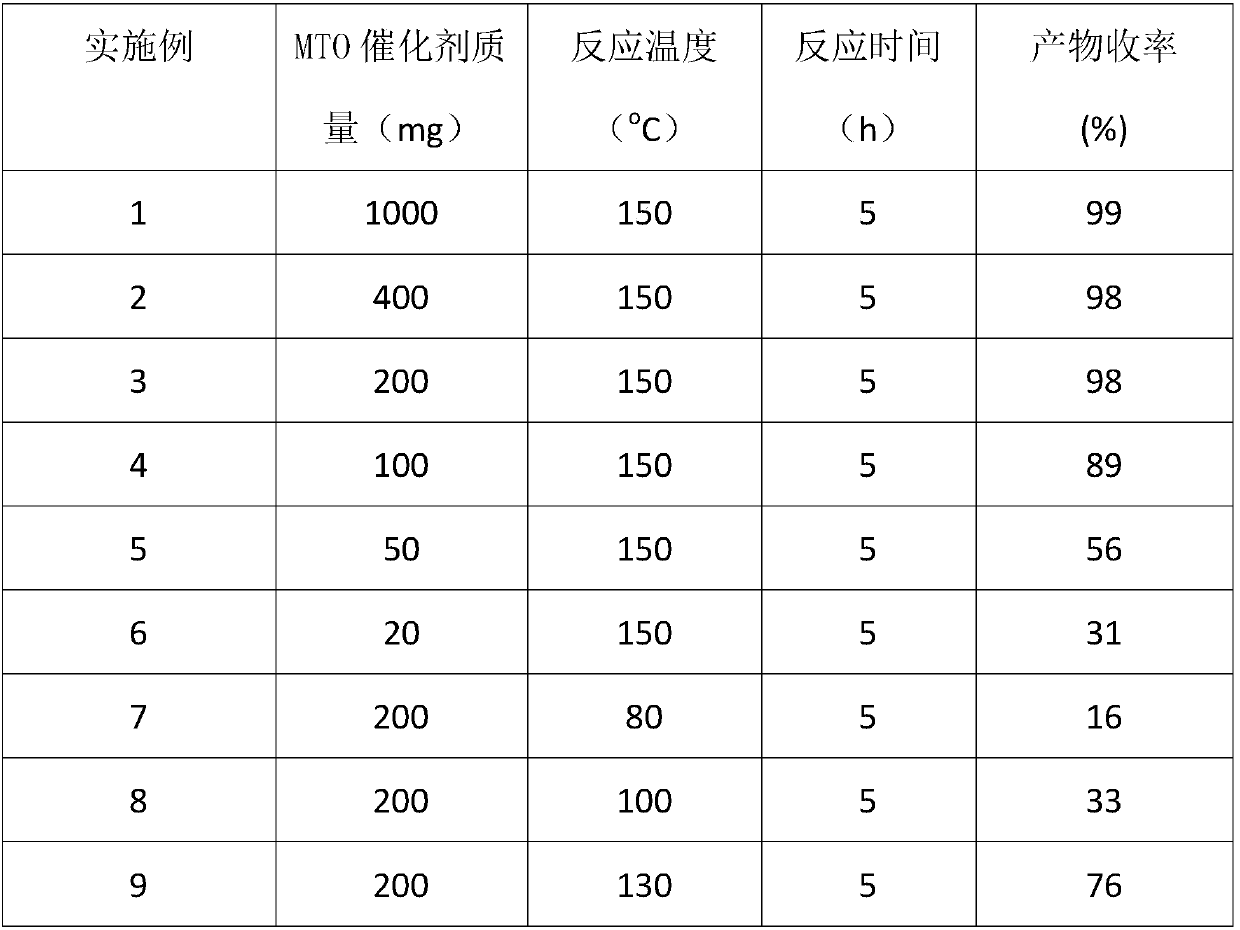
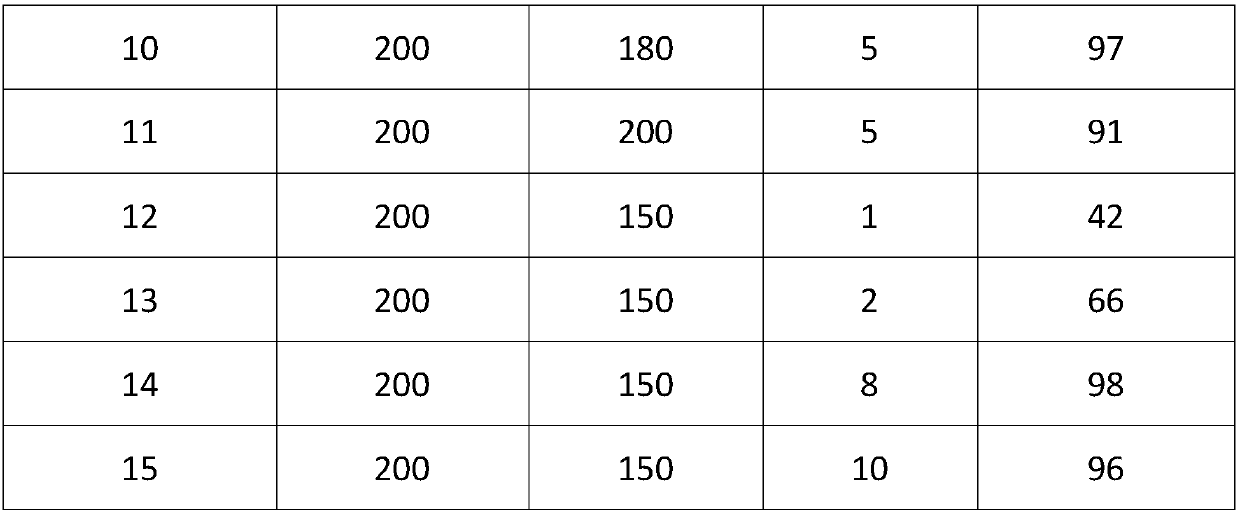
[0019] Add 2 grams of fructose, a certain amount of MTO and 20 grams of tetrahydrofuran into the reaction kettle respectively, seal it, stir and heat to the set reaction temperature, carry out stirring reaction at a speed of 1000 rpm for a certain period of time, and then stop the reaction. The reaction mixture sample was filtered, and the filtrate was analyzed by a liquid chromatograph through an ultraviolet detector. The reaction results are shown in Table 1.
[0020] Table 1. The reaction results of MTO catalyzing the conversion of fructose to HMF under different conditions in tetrahydrofuran solvent
[0021]
[0022]
[0023] The above examples show that: MTO in tetrahydrofuran solvent catalyzes the conversion of fructose to prepare HMF under the above different conditions and obtains good reaction results.
Embodiment 16~20
[0025] Add 2 grams of reaction substrate, 200 mg of catalyst MTO and 20 grams of tetrahydrofuran into the reaction kettle respectively, seal it and heat it to 150° C. with stirring at a speed of 1000 rpm for 5 hours, then stop the reaction. The reaction mixture sample was filtered, and the filtrate was analyzed by a liquid chromatograph through an ultraviolet detector. The reaction results are shown in Table 2.
[0026] Table 2. MTO-catalyzed conversion of different substrates to prepare HMF in tetrahydrofuran solvent
[0027] Example
[0028] Examples 16-20 show that the conversion of different substrates catalyzed by MTO to prepare HMF in tetrahydrofuran solvent has obtained excellent reaction results.
Embodiment 21~26
[0030] Add 2 grams of reaction substrate, 200 mg of catalyst MTO and 20 grams of reaction solvent into the reaction kettle, seal it and heat it to 150° C. with stirring at a speed of 1000 rpm for 5 hours, then stop the reaction. The reaction mixture sample was filtered, and the filtrate was analyzed by a liquid chromatograph through an ultraviolet detector. The reaction results are shown in Table 3.
[0031] Table 3. The reaction results of MTO-catalyzed conversion of fructose to HMF in different solvents
[0032] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com