Conjugated ligand-bridged ferrocene and ruthenium acetylene end group compound and preparation method and application thereof
A technology of ferrocene and compound, applied in the field of ferrocene and ruthenium acetylene terminal compound and its preparation, achieving the effect of broad application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of a conjugated ligand (phenyl) bridged ferrocene and ruthenium acetylene end group compound, the above conjugated ligand bridged ferrocene and ruthenium acetylene end group compound is 1,4-phenyl The bridged ferrocene and ruthenium acetylene terminal compound specifically comprises the following steps: under nitrogen protection, 0.34mmol pentamethylcyclopentadienyl (1,2-bisdiphenylphosphine ethane) ruthenium chloride Cp * Dissolve Ru(dppe)Cl (227mg), 0.28mmol 4-trimethylsilylethynylphenylferrocene (100mg), 5.58mmol potassium fluoride (321mg) in a mixed solution of 20ml methanol and 3-4ml tetrahydrofuran , the system was heated and refluxed for 24 hours, cooled to room temperature, filtered with suction, the solid was washed with 10ml of methanol and 10ml of n-hexane, and then separated by column chromatography. The eluent was acetone and petroleum ether with a volume ratio of 1:10, and finally separated 180 mg of yellow solid was obtained, yield 6...
Embodiment 2
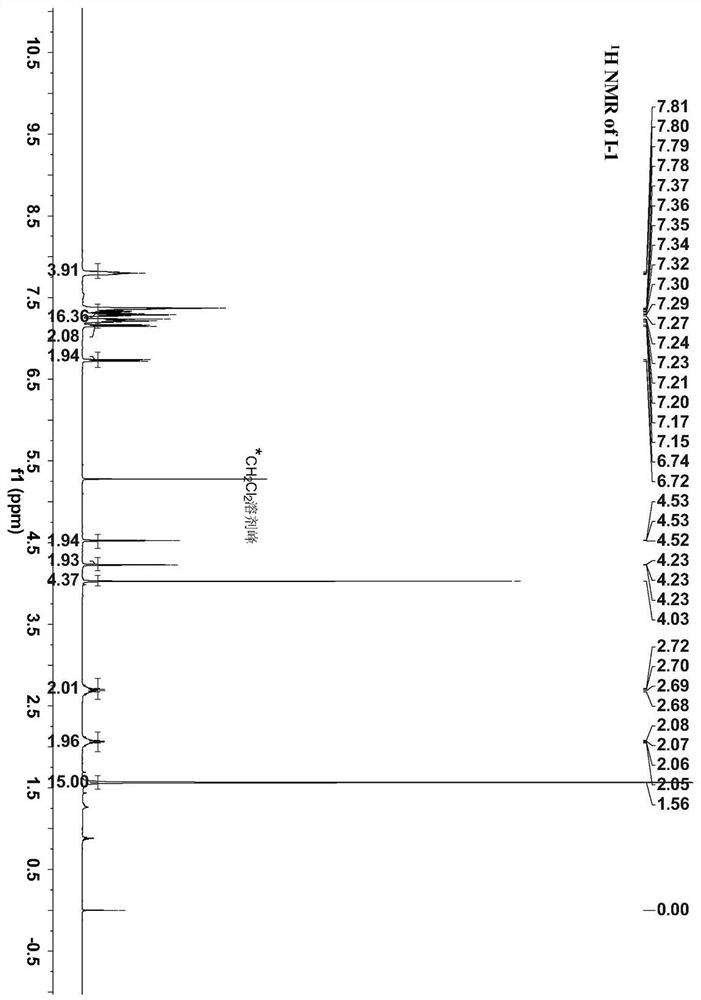
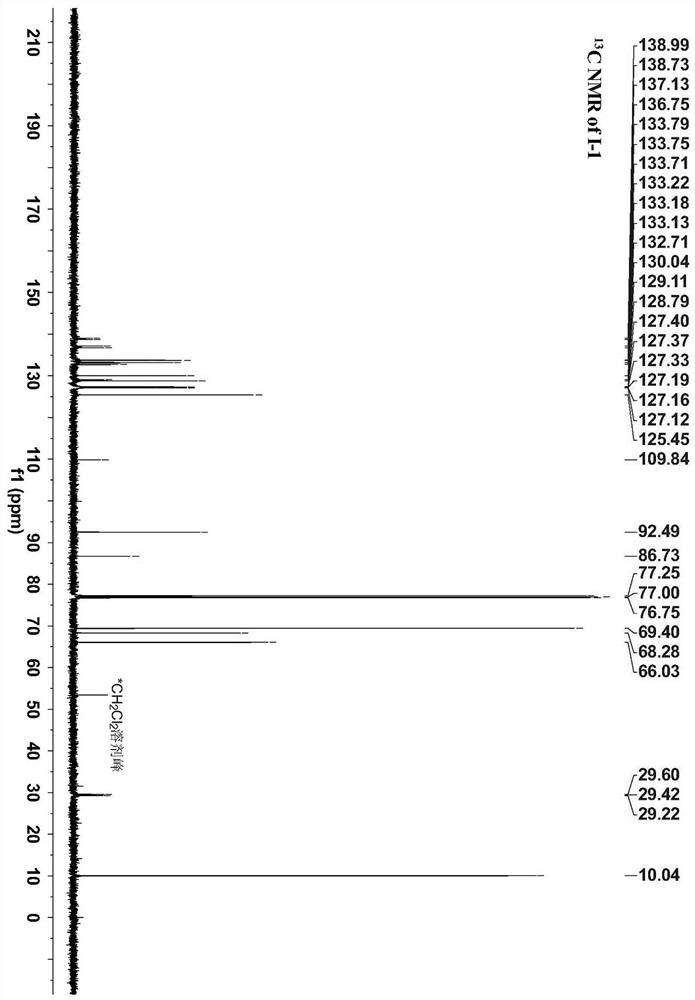
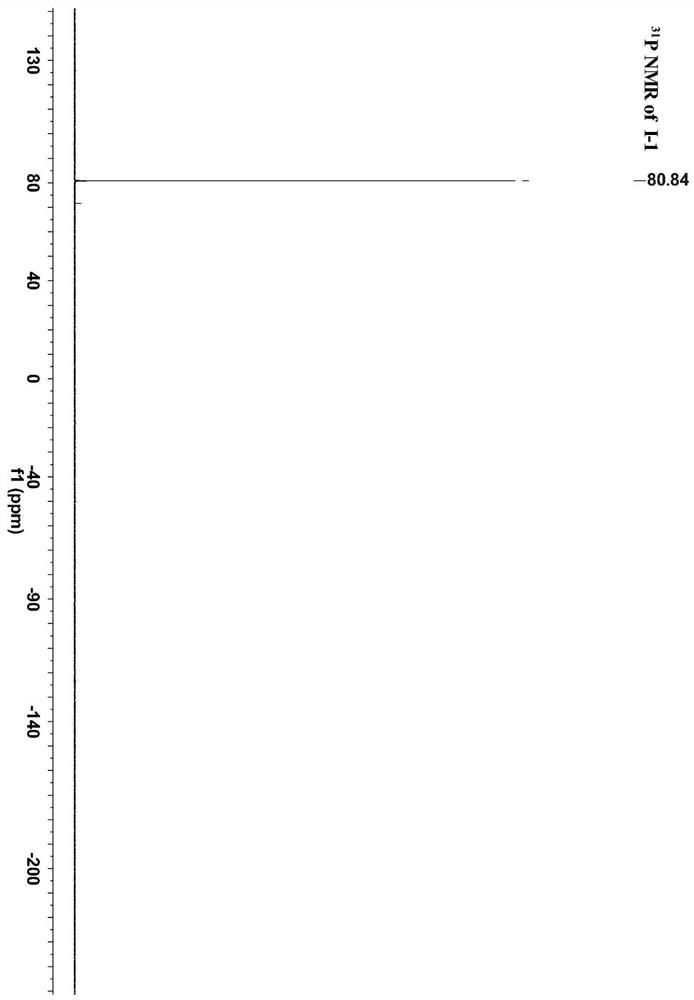
[0037] A method for preparing a ferrocene and ruthenium acetylene terminal compound bridged by a conjugated ligand (phenyl) is 1,3-benzene The ferrocene and ruthenium acetylene end group compound of radical bridging specifically comprise the following steps: under nitrogen protection, 0.34mmol pentamethylcyclopentadienyl (1,2-bisdiphenylphosphine ethane) chlorination Ruthenium Cp * Dissolve Ru(dppe)Cl (227mg), 0.28mmol 3-trimethylsilylethynylphenylferrocene (100mg), 5.58mmol potassium fluoride (321mg) in a mixed solution of 20ml methanol and 3-4ml tetrahydrofuran , the system was heated and refluxed for 24 hours, cooled to room temperature, filtered with suction, the solid was washed with 10ml of methanol and 10ml of n-hexane, and then separated by column chromatography. The eluent was acetone and petroleum ether with a volume ratio of 1:10, and finally separated 169 mg of pale yellow solid was obtained, yield 63%.
[0038]
[0039] The proton nuclear magnetic resonance spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com