Capecitabine impurity and preparation method thereof
A capecitabine and impurity technology, applied in the field of medicine, can solve the problems of capecitabine intermediate B not meeting quality standards, capecitabine being unable to continue production, affecting the normal production of capecitabine, and the like. The effect of increasing the washing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: confirm target impurity I structure
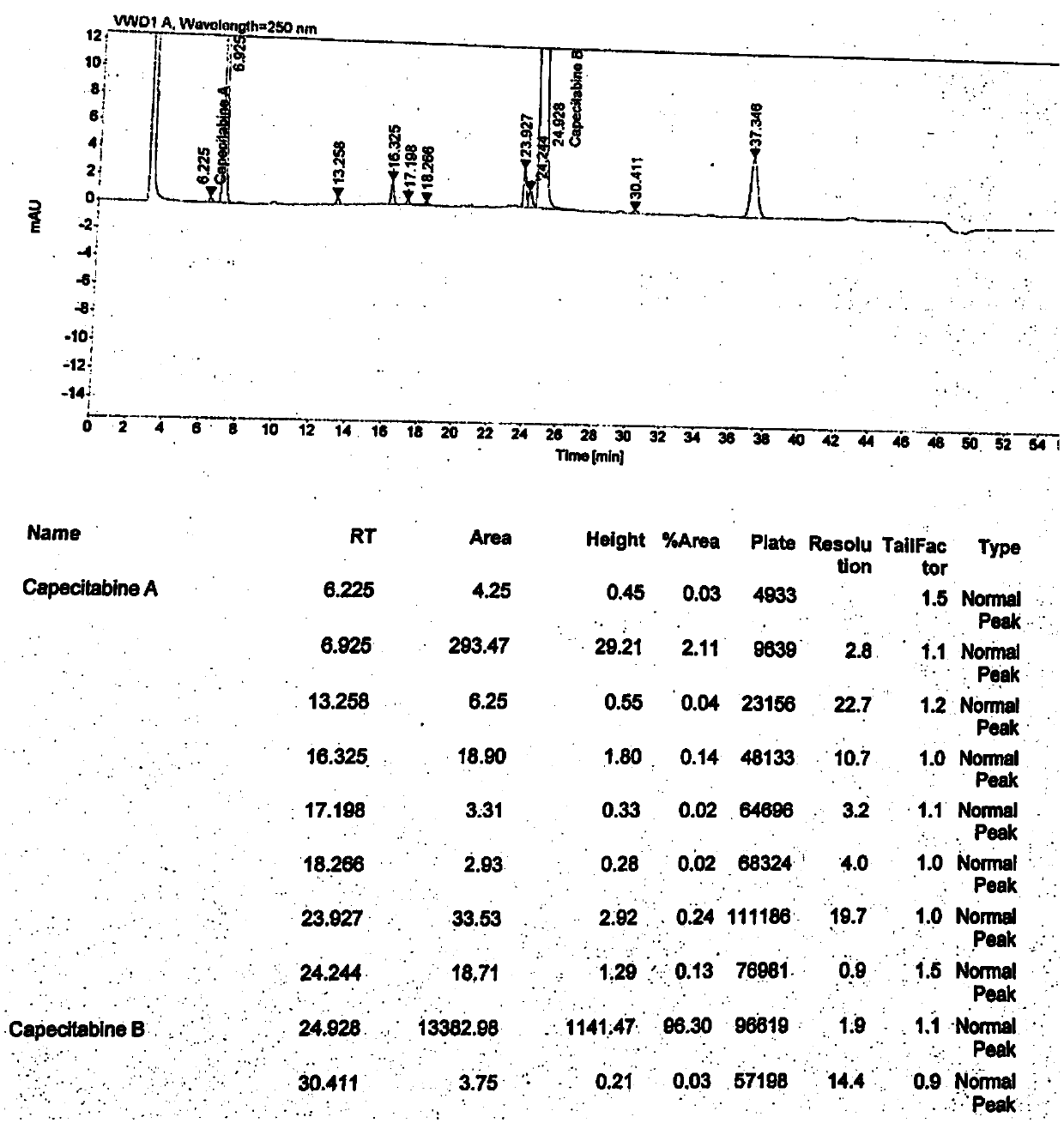
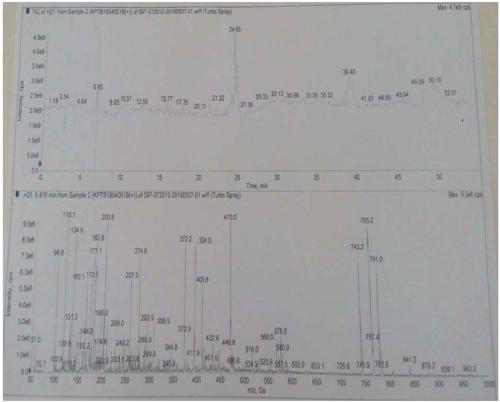
[0028] The capecitabine intermediate B was detected by liquid chromatography-mass spectrometry, and the results were as follows: figure 2 As shown, after inferring the possible structure of the target impurity I, the inferred structure has the following types:
[0029]
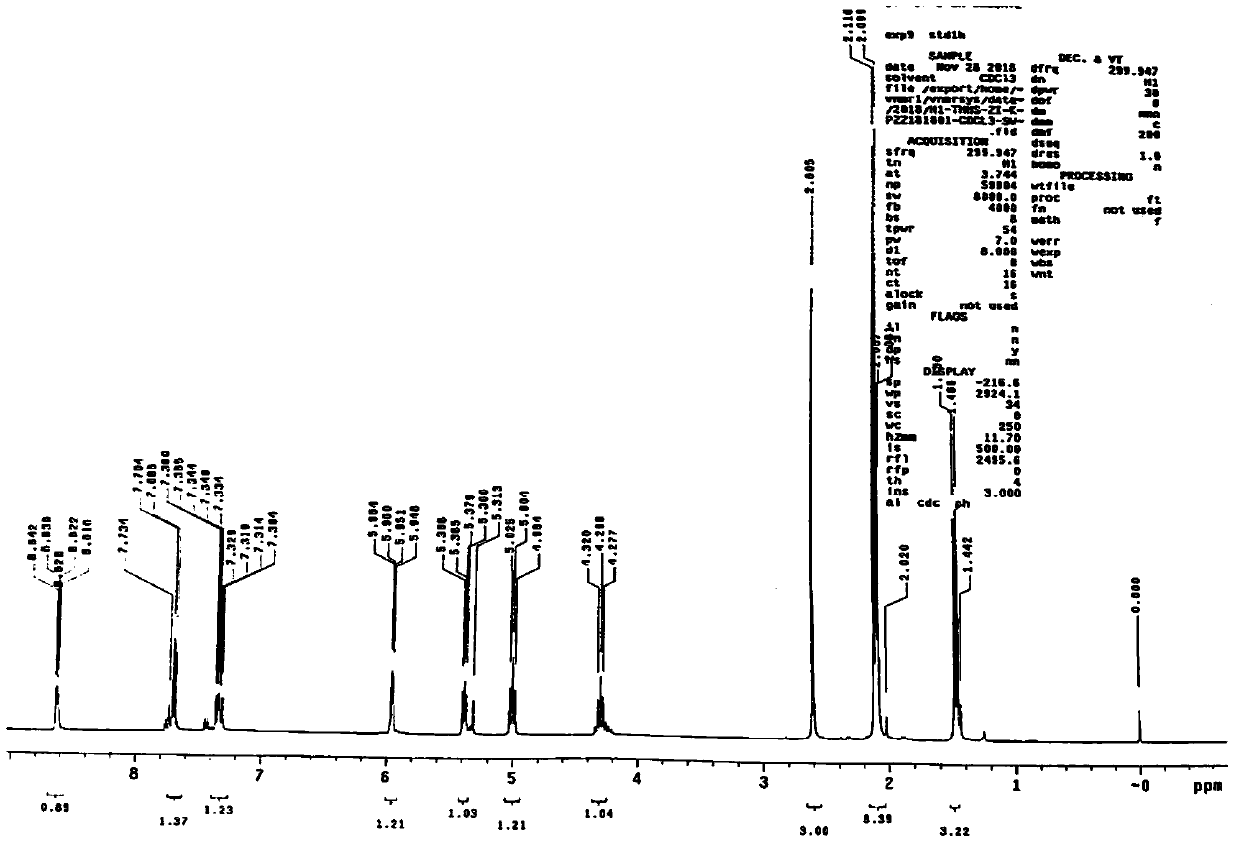
[0030] According to the reaction process of capecitabine intermediate B from 2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine (II) with n-pentyl chloroformate in the presence of pyridine and conditions, acetic acid will be generated during the synthesis process, and then combined with image 3 The hydrogen spectrum of impurity Ⅰ shown (with Figure 4 Intermediate B for comparison), confirming that structure 3 is impurity I, the chemical name is 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N 4 -(ethoxycarbonyl)cytidine.
Embodiment 2
[0032] Weigh 3.29g (0.01mol) of compound II into a 50ml three-neck flask, add 30ml of dichloromethane to dissolve, add 1.58g (0.02mol) of pyridine, cool down to 0°C, add 1.2g of glacial acetic acid (0.02mol) dropwise, During the addition process, the temperature should not exceed 5 ° C. After the dropwise addition, keep stirring for 1 hour. After the reaction is completed, add 10 ml of purified water and stir for 5 minutes. After standing still and layering, the obtained organic phase is subjected to vacuum distillation. After no liquid is evaporated, the obtained The oily substance is the impurity I, weighing 2.70 g, and the HPLC detection purity is 95.2%. Gained oil and intermediate A (used as a control) were added to the dichloride solution of capecitabine intermediate B, and the liquid phase was detected, and it was found that the content of impurity I (RRT=0.29) increased significantly (see attached Figure 5 ).
Embodiment 3
[0034] Weigh 3.29g (0.01mol) of compound II into a 50ml three-necked flask, add 30ml of dichloromethane to dissolve, add 2.37g (0.02mol) of pyridine, cool down to 0°C, add dropwise 0.9g of glacial acetic acid (0.015mol), dropwise During the addition process, control the temperature not to exceed 5°C. After the dropwise addition is completed, keep stirring for 1.5 hours. After the reaction is completed, add 10ml of purified water and stir for 5 minutes. Let stand and separate layers. The obtained organic phase is distilled under reduced pressure. After no liquid is evaporated, The obtained oily substance was impurity I, weighing 2.00 g, and its purity by HPLC was 93.2%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap