Kit for detecting minimal residual diseases (MRD)
A minimal residual disease and kit technology, applied in the biological field, can solve problems such as MRD false negatives, non-specific amplification, affecting the reliability and accuracy of MRD test results, and achieve the effect of predicting recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
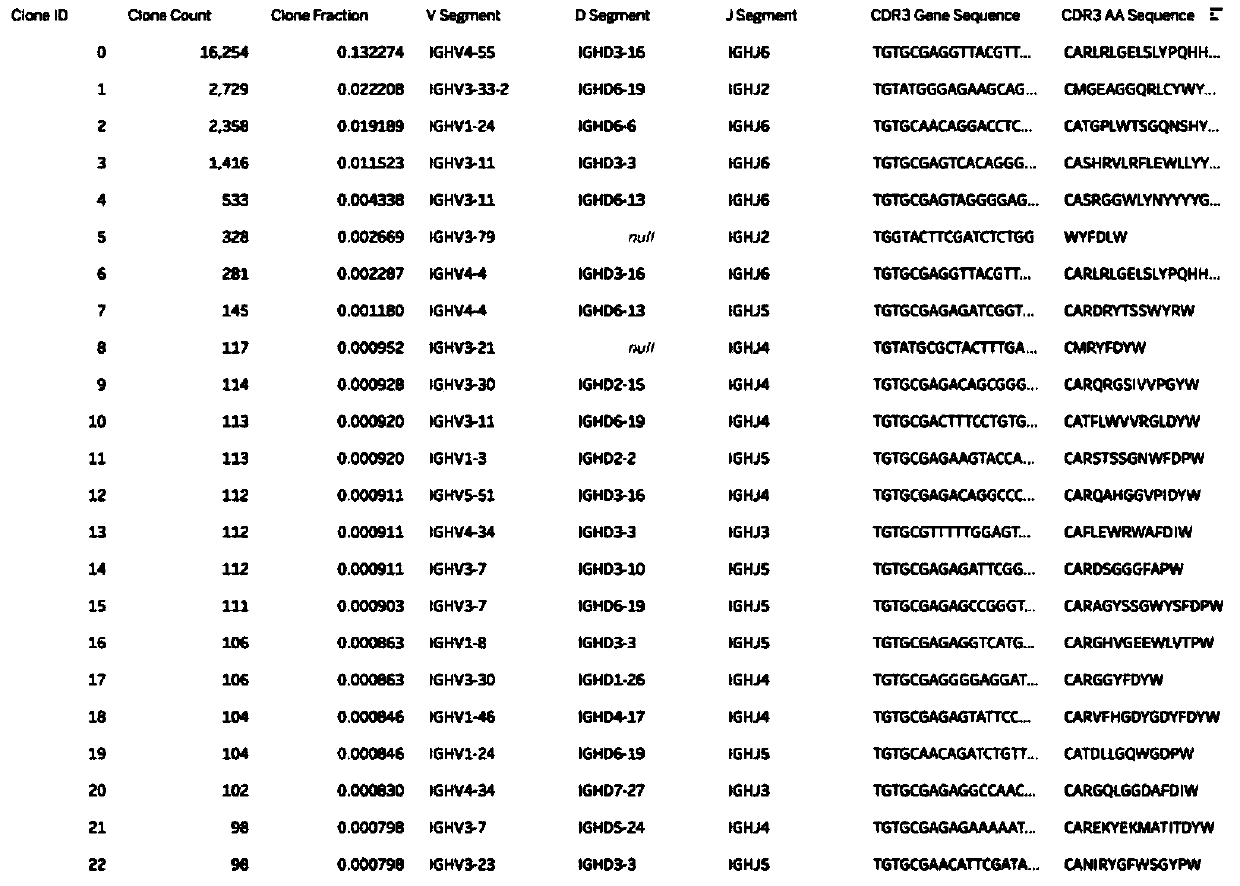
[0068] Example 1: Obtaining the sample genome
[0069] 1. According to before and after treatment and sample specificity, it can be divided into:
[0070] 100uL, 200uL, 300uL, 400uL, 500uL of human bone marrow samples before treatment in EDTA anticoagulant tubes;
[0071] Human bone marrow samples after treatment 100uL, 200uL, 300uL, 400uL, 500uL, 600uL, 700uL, 800uL, 900uL, 1mL, 2mL in EDTA anticoagulant tubes;
[0072] Human peripheral blood samples 5mL, 6mL, 7mL, 8mL, 9mL, 10mL in EDTA anticoagulant tubes.
[0073] 2. Use the red blood cell lysate to lyse the red blood cells in the sample and separate the nucleated living cells.
[0074] 3. The obtained nucleated viable cells were counted and then the genomic gDNA was extracted.
Embodiment 2
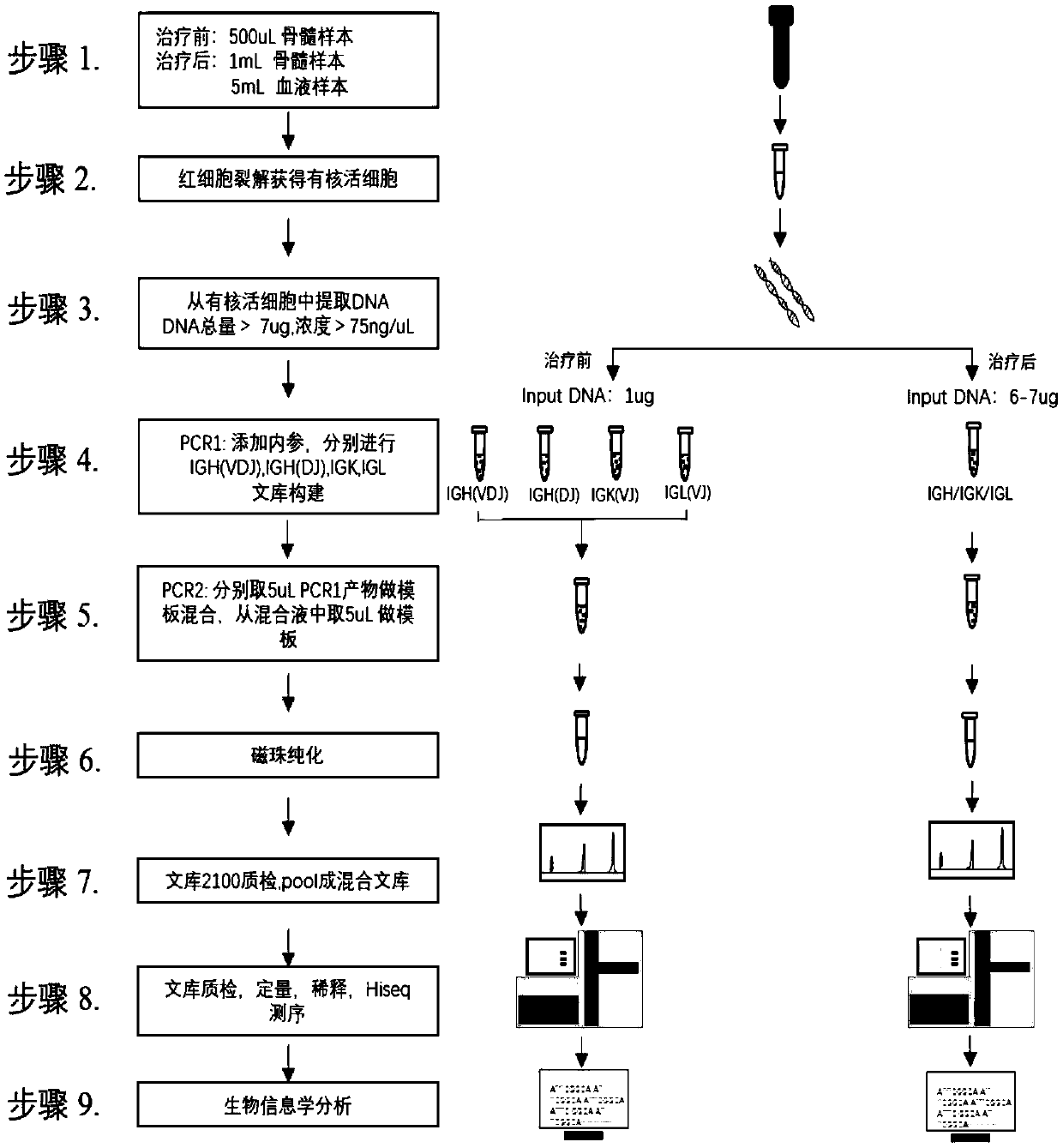
[0075] Example 2: Multiplex PCR amplification and library construction
[0076] Using the library construction kit in the kit, add primers of multiple pairs of V gene fragments and J gene fragments with UMB to the multiplex PCR reaction system, and add any three corresponding 1% input DNA templates of known amount. The sequenced internal reference DNA or House Keeping gene is specifically amplified simultaneously with the sample.
[0077] Primer set sequences are shown in Table 1
[0078] Table 1 Multiplex PCR Primers
[0079]
[0080]
[0081]
[0082]
[0083] The primer sequence structure is as figure 2 As shown, wherein the Read1 sequence is SEQ ID NO:248, and the Read2 sequence is SEQ ID NO:249.
[0084] Wherein the sequence of the internal reference DNA is shown in SEQ ID NO: 128-226.
[0085] Wherein the PCR primer sequence for amplifying the House Keeping gene is shown in SEQ ID NO: 250-253.
[0086] Multiplex PCR system, including 25μL, 50μL, the fol...
Embodiment 3
[0092] Example 3: High-throughput sequencing and bioinformatics analysis
[0093] The method of the present invention uses the Hiseq system from Illumina Company, and Hiseq is a sequencing-by-synthesis technology based on single-molecule clusters, based on a proprietary reversible termination chemical reaction principle. During sequencing, random fragments of DNA are attached to the optically transparent glass surface (Flow cell). After extension and bridge amplification, these DNA fragments form hundreds of millions of clusters (clusters) on the Flow cell. Each cluster has Single-molecule clusters of thousands of copies of the same template. Then, the template DNA to be tested is sequenced by reversibly terminated sequencing by synthesis (Sequencing by Synthesis, SBS) technology using four special deoxyribonucleotides with fluorescent groups.
[0094] First, add the on-machine sequencing primers and tags of the Illumina high-throughput sequencer to the two ends of the PCR1 p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com