Primer for rapidly detecting respiratory tract adenoviruses based on RAA fluorescent method, probe and detection kit
A detection kit and respiratory technology, applied in the field of molecular biology, can solve the problems of complex primer design, high false positive rate, and low sensitivity, and achieve the effects of convenient, rapid and accurate identification, high sensitivity, and reduced detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
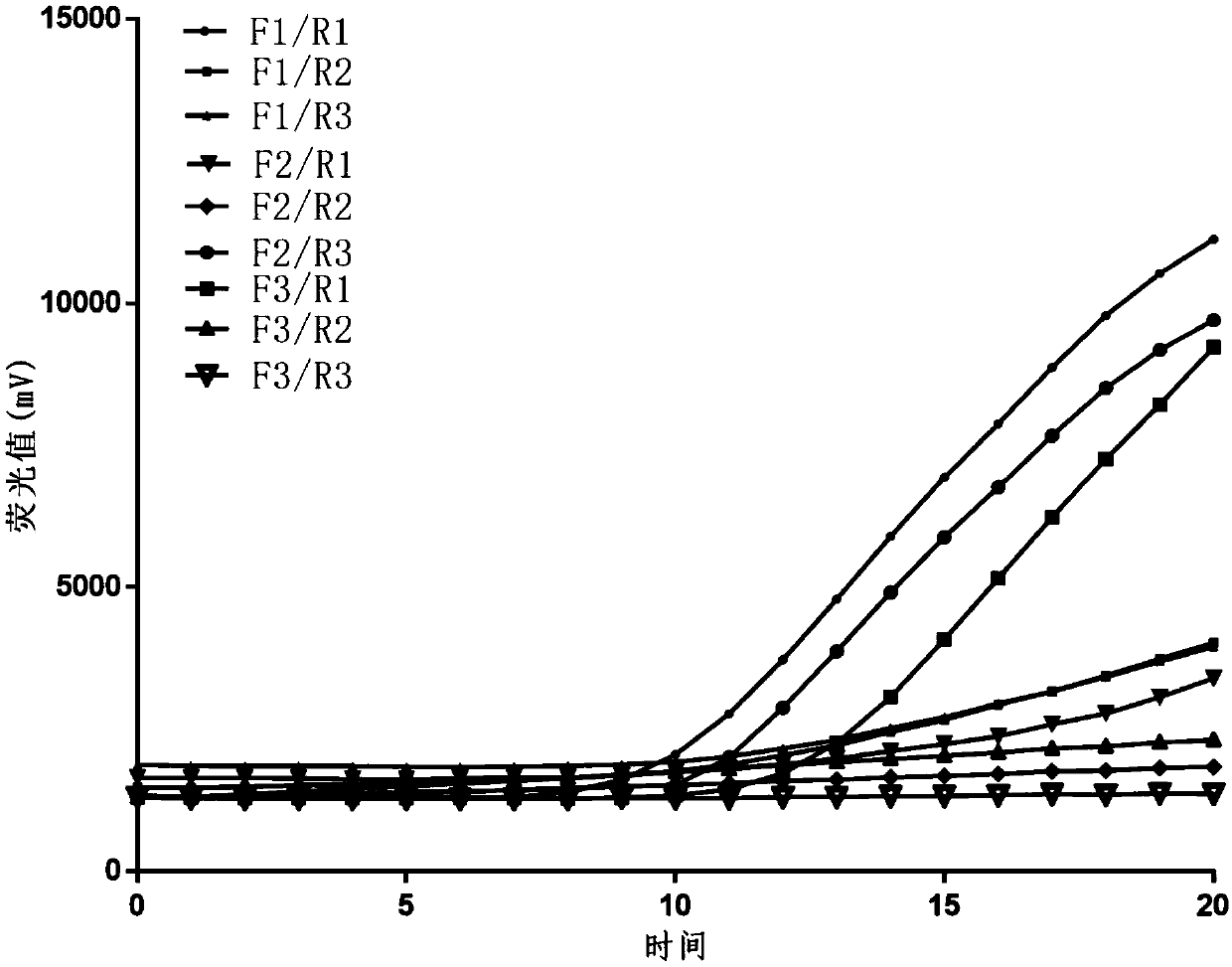
[0081] Embodiment 1: Primer screening experiment
[0082] Upstream primers:
[0083] F1: 5'-CAGCATCAGTTTCACGAGCATCAACCTCTATGC-3';
[0084] F2: 5'-GCATCAGTTTCACGAGCATCAACCTCTATGCTA-3';
[0085] F3: 5'-CATCAGTTTCACGAGCATCAACCTCTATGCTAC-3';
[0086] Downstream primers:
[0087] R1: 5'-TGCGAGAAGGAATGGAAATGGGAATATTGGTTGC-3';
[0088] R2: 5'-CGAGAAGGAATGGAAATGGGAATATTGGTTGCAT-3';
[0089] R3: 5'-GAGAAGGAATGGAAATGGGAATATTGGTTGCATT-3';
[0090] The probe sequence is:
[0091] AGCCATGCTGCGGAATGACACCAATGATCAGTCATTCAACGACTAYCTATC
[0092] The probe is modified with a fluorescent reporter group (FAM) and a fluorescent quencher group (BHQ1);
[0093] The modified probe is
[0094] AGCCATGCTGCGGAATGACACCAATGATCAG(FAM-dT)C(THF)(BHQ1-dT)TCAACGACTAYCTATC;
[0095] The composition of the kit is shown in Table 1, and Table 1 is the composition list of the kit:
[0096] Table 1
[0097]
[0098] The prepared recombinant plasmid working standards are:
[0099] Working Standard 1, con...
Embodiment 2
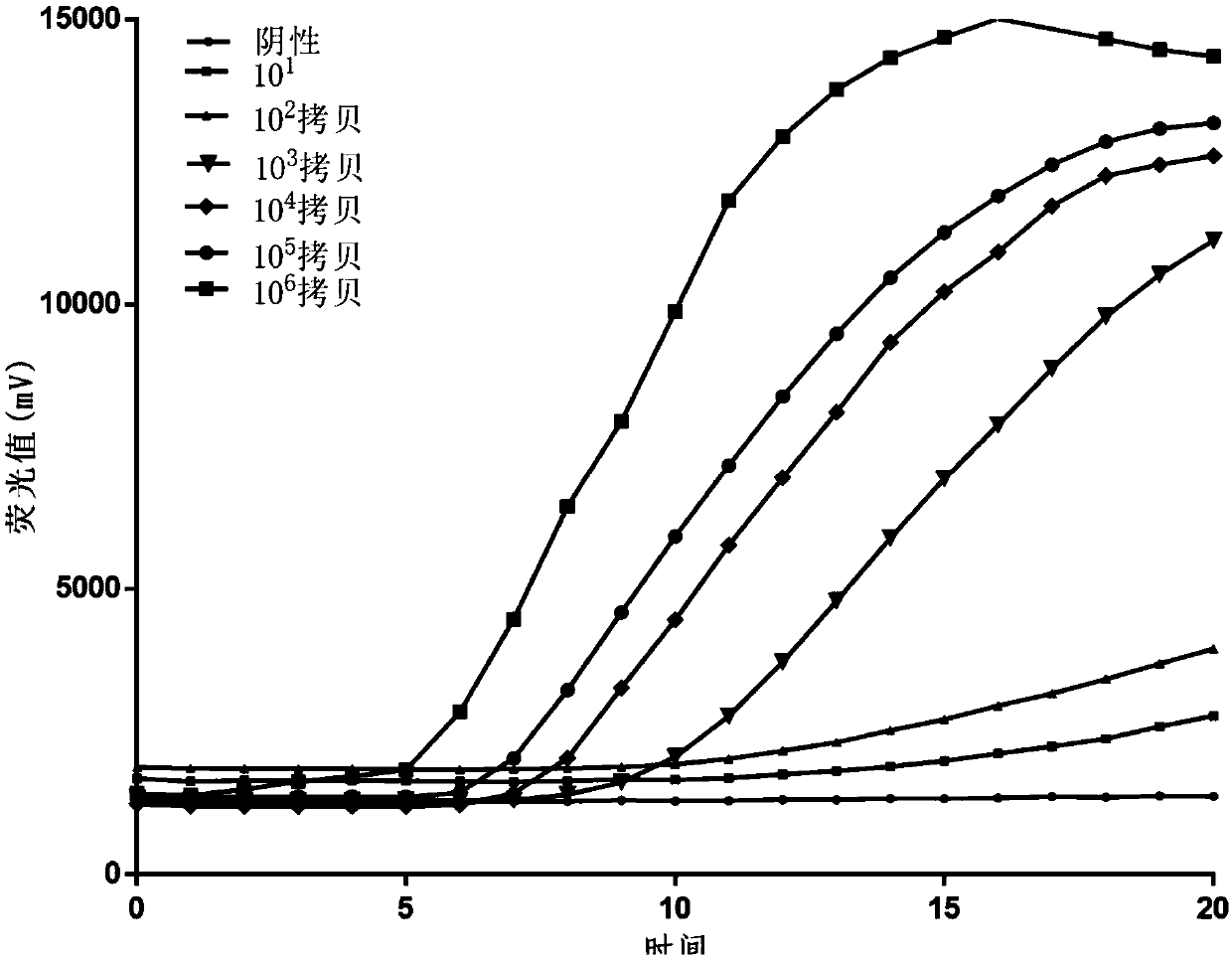
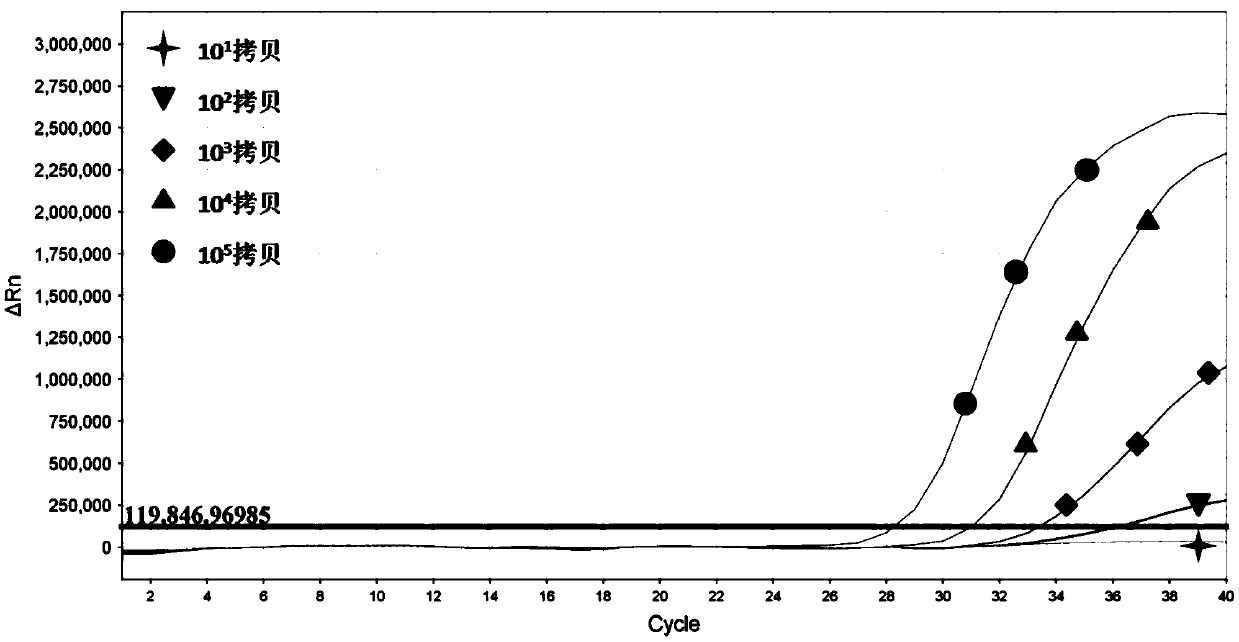
[0107] Embodiment 2: Sensitivity experiment
[0108] Upstream primers:
[0109] 5'-CAGCATCAGTTTCACGAGCATCAACCTCTATGC-3'
[0110] Downstream primers:
[0111] 5'-TGCGAGAAGGAATGGAAATGGGAATATTGGTTGC-3'
[0112] The probe sequence is:
[0113] AGCCATGCTGCGGAATGACACCAATGATCAGTCATTCAACGACTAYCTATC
[0114] The probe is modified with a fluorescent reporter group (FAM) and a fluorescent quencher group (BHQ1);
[0115] The modified probe is:
[0116] AGCCATGCTGCGGAATGACACCAATGATCAG(FAM-dT)C(THF)(BHQ1-dT)TCAACGACTAYCTATC;
[0117] The composition of the kit is shown in Table 1.
[0118] The prepared recombinant plasmid working standards are:
[0119] Working Standard 1, containing 0.2 × 10 6 Copies / ul respiratory adenovirus plasmid non-infectious DNA fragment.
[0120] Working Standard 2, containing 0.2 x 10 5 Copies / ul respiratory adenovirus plasmid non-infectious DNA fragment.
[0121] Working Standard 3, containing 0.2 × 10 4 Copies / ul respiratory adenovirus plasmid non-i...
Embodiment 3
[0136] Embodiment 3: repeatability experiment
[0137] The sequences of the primer probe and the positive quality control product are the same as those in Example 1.
[0138] The composition of the kit is shown in Table 1.
[0139] The implementation method of the repeat experiment is:
[0140] (1) Reaction buffer preparation:
[0141] Draw 173 μL of reaction buffer from the reaction buffer tube in the kit and add it to the pre-prepared 1.5ml PE tube, then add 8 μL of the mixture of probe and primer (the concentration of the probe is 0.02mmol / , the concentration of the primer is 0.03mM) , and mix well to obtain the mixed reaction buffer.
[0142] (2) RAA fluorescent basic reaction reagent redissolved
[0143] Prepare 4 RAA fluorescent basic reaction reagents, each time draw 45 μL of the reaction buffer mixed in step 1 and add them to the prepared 4 RAA fluorescent basic reaction reagent tubes, so that the lyophilized powder is fully dissolved and mixed to become RAA React...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com