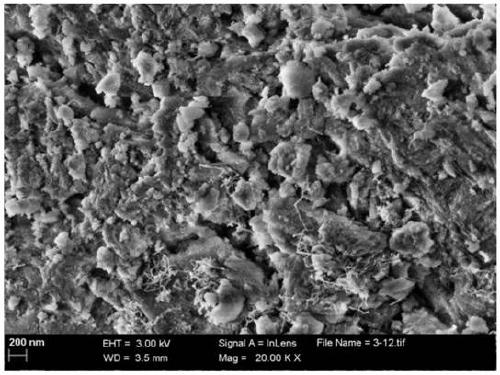
Dual-pore nickel based catalyst, preparation method thereof and application thereof in methanol carbon dioxide reforming reaction
A nickel-based catalyst and nickel source technology, applied in the field of porous materials, can solve the problems of unsuitability for large-scale production and high preparation costs, and achieve the effects of solving the problem of carbon deposition, low cost, and improving catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention provides a kind of preparation method of biporous nickel-based catalyst, comprising the following steps:
[0029] (1) After mixing triblock copolymer P123, nickel source, and aluminum source in an organic solvent, add concentrated nitric acid, and then add polystyrene microsphere dispersion liquid to obtain a mixed material liquid;
[0030] (2) Drying and calcining the mixed material liquid obtained in the step (1) in sequence to obtain a dual-porous nickel-based catalyst.
[0031] All raw materials of the present invention are commercially available goods.
[0032] In the invention, after mixing the triblock copolymer P123, nickel source and aluminum source in an organic solvent, concentrated nitric acid is added, and then polystyrene microsphere dispersion liquid is added to obtain a mixed material liquid. In the present invention, the nickel source preferably includes nickel nitrate, and the aluminum source preferably includes aluminum isopropoxide. I...
Embodiment 1
[0048] Weigh 3.0125g of tri-block copolymer P1233, dissolve in 60.0mL of absolute ethanol, stir until dissolved, weigh 0.4836g of nickel nitrate and dissolve in the above solution and continue to stir, then weigh 6.1236g of aluminum isopropoxide and dissolve in the solution Stirring was continued in the middle, and 4.5mL concentrated nitric acid was added dropwise and vigorously stirred for 5h. Add 3.0 mL of polystyrene microsphere dispersion (10% solid content) to the above solution, and continue stirring for 5 min. Dry at 60°C for 3 days and calcined at 700°C for 4h to prepare a dual-porous nickel-based catalyst, abbreviated as 6%Ni-M100-MA-EISA, in which the nickel loading is 6wt.%.
Embodiment 2
[0050] Weigh 3.1628g of triblock copolymer P1233.1628g, dissolve in 60.0mL of absolute ethanol, stir until dissolved, weigh 0.3186g of nickel nitrate and dissolve in the above solution and continue stirring, then weigh 6.1205g of aluminum isopropoxide and dissolve in the solution Stirring was continued in the middle, and 4.5mL concentrated nitric acid was added dropwise and vigorously stirred for 5h. Add 3.0 mL of polystyrene microsphere dispersion (50% solid content) to the above solution, and continue stirring for 5 min. Dry at 60°C for 3 days and calcined at 700°C for 4h to prepare a dual-porous nickel-based catalyst, abbreviated as 4%Ni-M100-MA-EISA, in which the nickel loading is 4wt.%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com