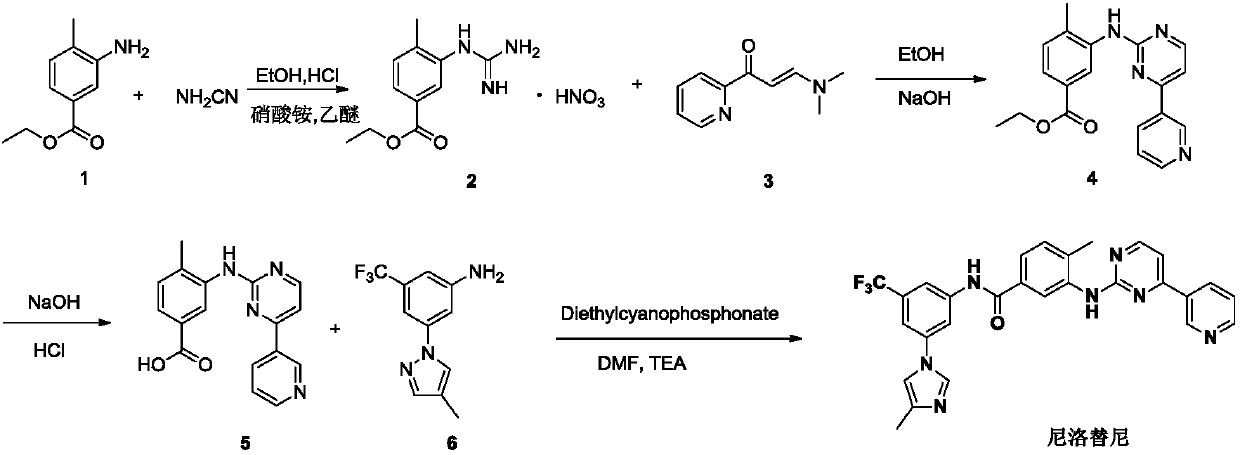
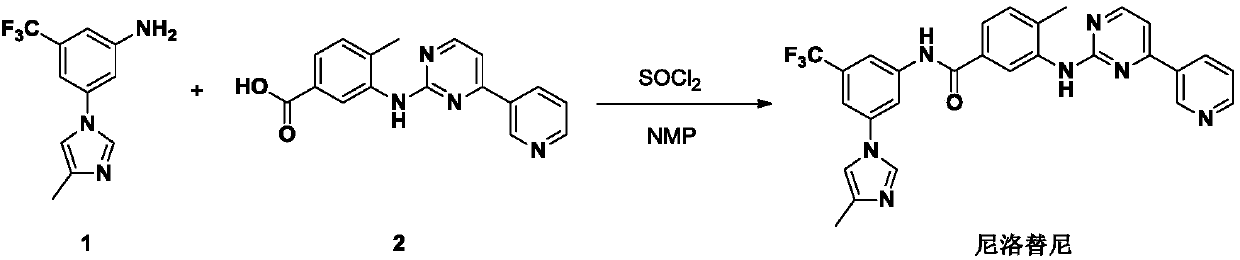
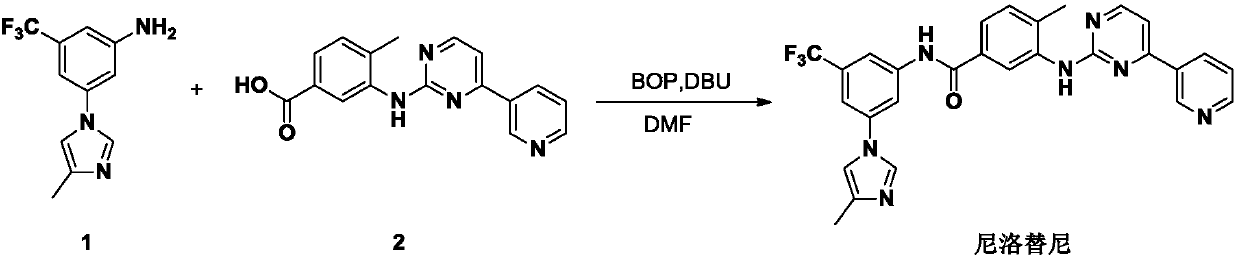
Preparation method and intermediate of nilotinib
A nilotinib, volumetric technology, applied in the field of drug synthesis, can solve the problems of many side reactions, expensive reagents, low product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Add respectively 3-amino-4-methylbenzoic acid methyl ester (16.5 grams, 0.10mol, 1.0 equivalents), di-tert-butyl dicarbonate (Boc 2 O, 21.8 g, 0.10 mol, 1.0 equivalent) and 100 ml of n-butanol. The reaction mixture was heated to 50-55° C. and stirred for 5-8 hours. High-efficiency liquid chromatography monitors that the reaction is complete. After adding 1000 grams of water to the reaction mixture, the system is concentrated to 100 milliliters. After the concentration is completed, it is filtered, and the filter cake is dried to obtain the nilotinib intermediate 3-((tert-butoxycarbonyl)amino) -25.5 g of methyl 4-methylbenzoate (Intermediate A), white to yellow solid, molar yield 96%.
Embodiment 2
[0085] Add respectively 3-amino-4-methylbenzoic acid methyl ester (33 grams, 0.2mol, 1.0 equivalents), di-tert-butyl dicarbonate (Boc2 O, 65.5 g, 0.3 mol, 1.5 equiv) and methanol 200 ml. The reaction mixture was heated to 60-65° C. and stirred for 6-10 hours. High-efficiency liquid chromatography monitors that the reaction is complete. After adding 3000 grams of water to the reaction mixture, the system is concentrated to 200 milliliters. After the concentration is completed, it is filtered, and the filter cake is dried to obtain the nilotinib intermediate 3-((tert-butoxycarbonyl)amino) - 47.8 g of methyl 4-methylbenzoate (Intermediate A), white to yellow solid, molar yield 90%.
Embodiment 3
[0087] Add respectively 3-amino-4-methylbenzoic acid methyl ester (16.5 grams, 0.1mol, 1.0 equivalents), di-tert-butyl dicarbonate (Boc 2 O, 24.0 g, 0.11 mol, 1.1 equiv) and 100 ml of ethanol. The reaction mixture was heated to 55-60° C. and stirred for 6-8 hours. High-efficiency liquid chromatography monitors that the reaction is complete. After adding 1000 grams of water to the reaction mixture, the system is concentrated to 100 milliliters. After the concentration is completed, it is filtered, and the filter cake is dried to obtain the nilotinib intermediate 3-((tert-butoxycarbonyl)amino) - 25.4 g of methyl 4-methylbenzoate (Intermediate A), white to yellow solid, molar yield 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com