Application of thiazolopyranone analogues in preparation of anti-inflammatory medicines
A technology of anti-inflammatory drugs and analogs, applied in the directions of anti-inflammatory agents, drug combinations, antipyretics, etc., can solve the problems of inability to increase polar excretion, lack of fatty chains, and difficult to metabolize, and achieve the effect of significant anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
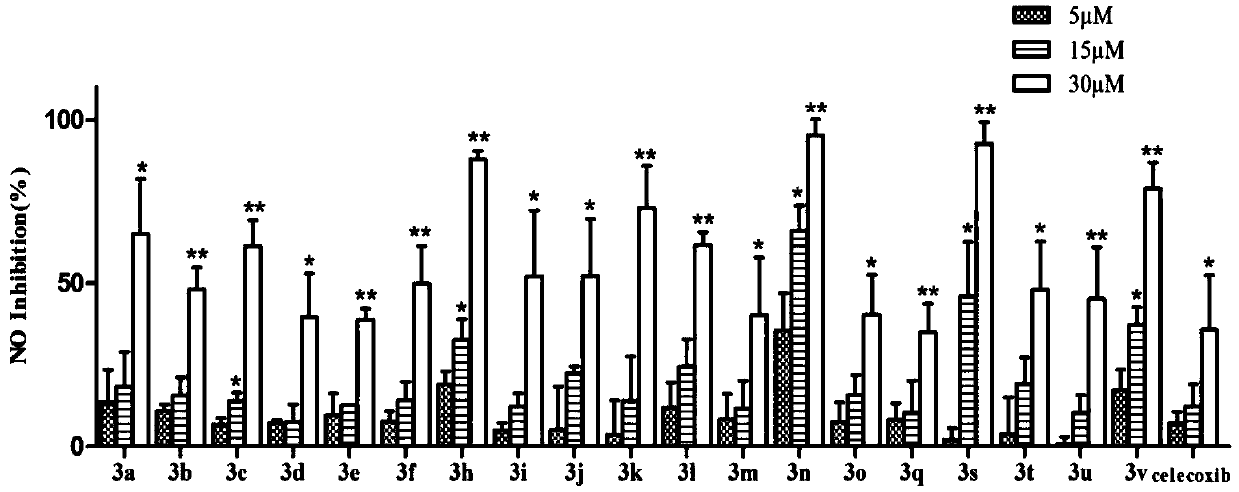
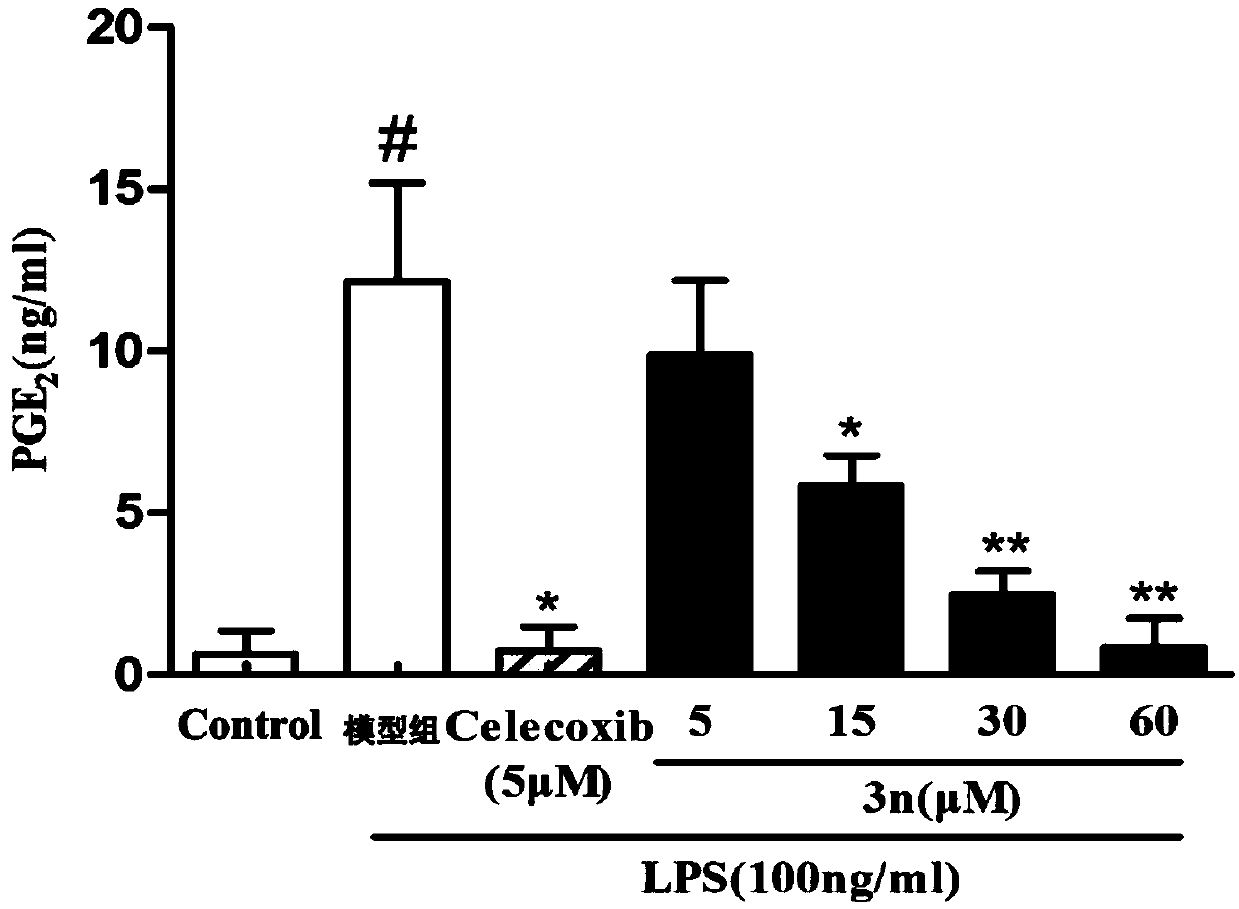
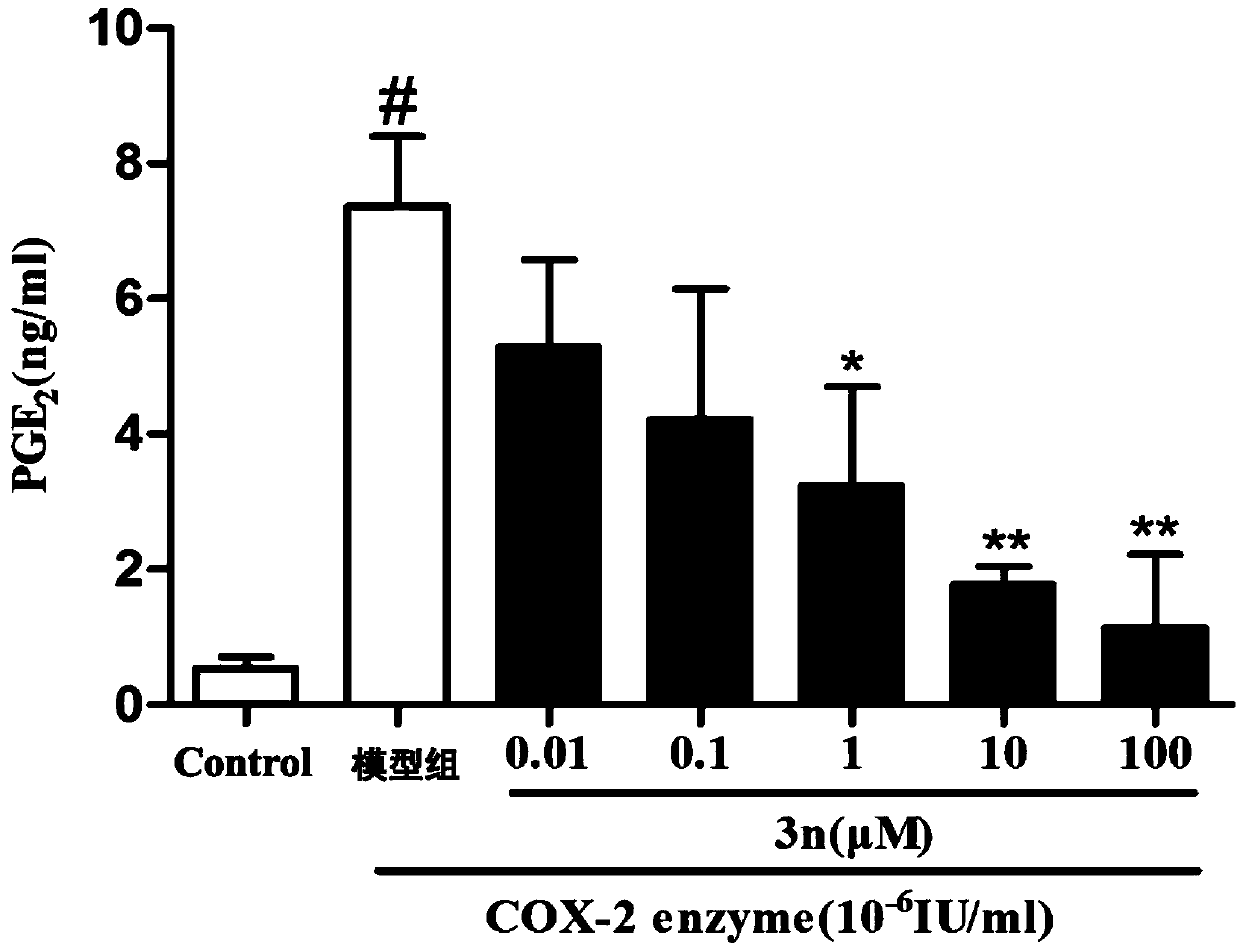
[0037] The synthesis and anti-inflammatory activity of the thiazolopyrone analogs of the present invention will be described in detail below in conjunction with specific examples. It should be understood that these examples are used to illustrate the present invention and not to limit the scope of the present invention.
[0038] 1. Structure and synthesis of thiazolopyrone analogs
[0039] In Examples 1-23, the structures of thiazolopyrone analogues are as follows:
[0040]
[0041] According to different configurations, the synthesis method of the above-mentioned chiral thiazolopyrone analogs can be selected, and according to different compounds, attention should be paid to the selection of the corresponding 5-enthiazolone substrate during the synthesis process. In Examples 1-23, the configurations and NMR characterizations of the thiazolopyrone analogs are shown in Table 1.
[0042]Table 1 Configuration and NMR characterization of thiazolopyrone analogues in Examples 1-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com