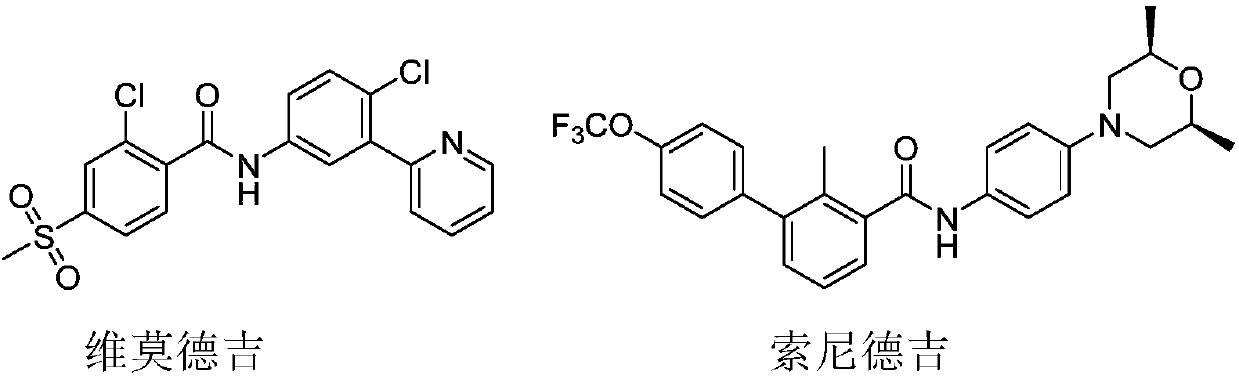
Hedgehog pathway inhibitor
A technology of hedgehog pathway and solvent medium, applied in the field of hedgehog pathway inhibitors, can solve the problem of lack of effective drugs for medulloblastoma, and achieve the effect of good growth and inhibition of growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The abbreviation HATU in this embodiment refers to the compound of the following formula I structure
[0036] Formula (I)
[0037]
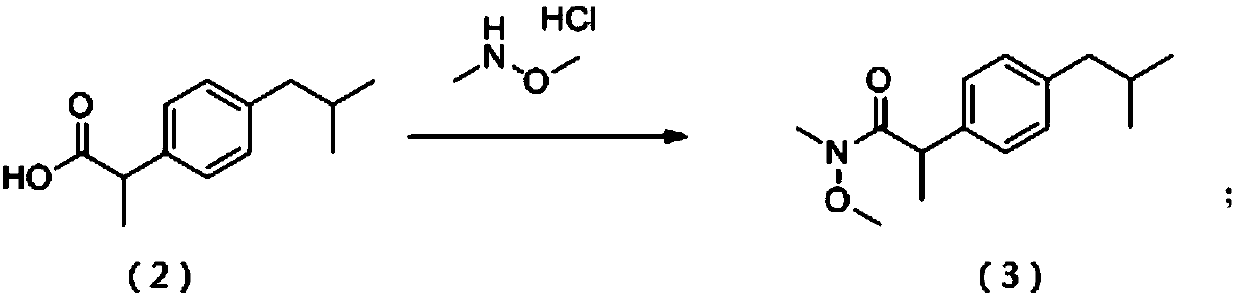
[0038] (1) 2-(4-isobutylphenyl)propionic acid (formula (2), 10g, 49mmol) was placed in tetrahydrofuran (100mL), and dimethylhydroxylamine hydrochloride (5g, 49mmol) was added at room temperature, Triethylamine (15g, 150mmol) and HATU (23g, 60mmol), the reaction mixture was stirred at room temperature for 16 hours, concentrated, and was purified by silica gel column (mobile phase PE (petroleum ether):EA (ethyl acetate)=20:1) Purification gave the product 2-(4-isobutylphenyl)-N-methoxy-N-methylpropanamide (formula (3), 10 g, 40 mmol) as a colorless oil. Formula (3)LCMS(ESI):m / z250.1[M+1] + .
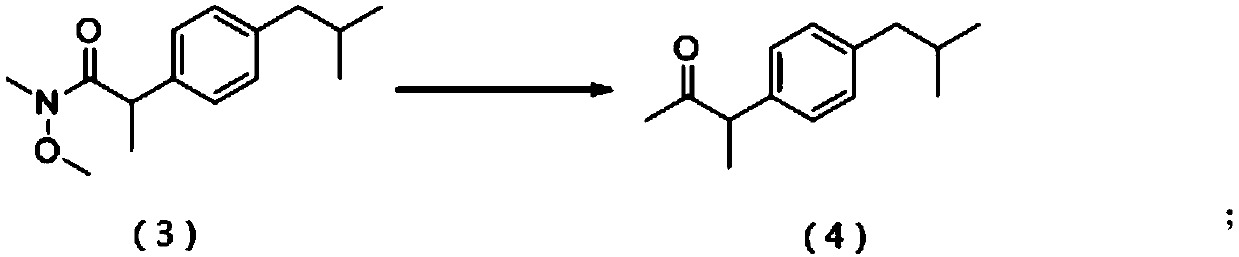
[0039] (2) 2-(4-isobutylphenyl)-N-methoxy-N-methylpropionamide (formula (3), 11g, 44.2mmol) obtained in step (1) was added to tetrahydrofuran (200mL ), at 0°C, methyl Grignard reagent (14.7mL, 3mol / L) was added dropwise, after the dropwise add...
Embodiment 2
[0043]
[0044] According to the method described in Embodiment 1, the difference between the method described in Embodiment 2 and the method described in Embodiment 1 is that it also includes the following steps (5):
[0045] (5) Place the compound (0.35g, 0.83mmol) of the formula (1-1) obtained in step (4) in Example 1 in dichloromethane (20ml), add tert-butylmercaptan (720mg), and react Add aluminum trichloride at 0°C, react for 2 hours and then gradually raise the temperature to room temperature. After quenching with water, extract with dichloromethane, concentrate and purify by preparative chromatography to obtain the oily product formula (1-2) (0.3g, 0.72 mmol). Formula (1-2)LCMS(ESI):m / z418.3[M+1] + ; 1 H NMR (400MHz, DMSO): δ (ppm) 0.88 (s, 3H) 0.90 (s, 3H) 1.81 (s, 3H) 1.81-1.88 (m, 1H), 2.44 (d, 2H), 5.72 (s, 2H) 6.75 (d, 2H) 6.93 (m, 2H) 7.10-7.22. (m, 6H) 7.37 (m, 1H) 7.41 (m, 1H) 7.47 (m, 1H).
Embodiment 3
[0047] This example is used to illustrate the effect of the compound of formula (1) of the present invention on tumor proliferation associated with the activation of hedgehog pathway.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com